Article Text
Abstract
Background: Dementia with Lewy bodies (DLB) is one of the main differential diagnoses of Alzheimer's disease (AD). Key pathological features of patients with DLB are not only the presence of cerebral cortical neuronal loss, with Lewy bodies in surviving neurones, but also loss of nigrostriatal dopaminergic neurones, similar to that of Parkinson's disease (PD). In DLB there is 40–70% loss of striatal dopamine.
Objective: To determine if detection of this dopaminergic degeneration can help to distinguish DLB from AD during life.
Methods: The integrity of the nigrostriatal metabolism in 27 patients with DLB, 17 with AD, 19 drug naive patients with PD, and 16 controls was assessed using a dopaminergic presynaptic ligand, 123I-labelled 2β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl)nortropane (FP-CIT), and single photon emission tomography (SPET). A SPET scan was carried out with a single slice, brain dedicated tomograph (SME 810) 3.5 hours after intravenous injection of 185 MBq FP-CIT. With occipital cortex used as a radioactivity uptake reference, ratios for the caudate nucleus and the anterior and posterior putamen of both hemispheres were calculated. All scans were also rated by a simple visual method.
Results: Both DLB and PD patients had significantly lower uptake of radioactivity than patients with AD (p<0.001) and controls (p<0.001) in the caudate nucleus and the anterior and posterior putamen.
Conclusion: FP-CIT SPET provides a means of distinguishing DLB from AD during life.
- FP-CIT single photon emission tomography
- dementia with Lewy bodies
- Alzheimer's disease
- DLB, dementia with Lewy bodies
- AD, Alzheimer's disease
- PD, Parkinson's disease
- SPET, single photon emission tomography
- FP-CIT, 2β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl)nortropane
- MMSE, mini mental state examination
- CAMCOG, Cambridge cognitive function examination
- CDR, clinical dementia rating
- BEHAVE-AD, behavioural pathology in Alzheimer's disease
- UPDRS, unified Parkinson's disease rating scale
- H&Y stage, Hoehn and Yahr stage
- CAPE, Clifton assessment procedure for the elderly
Statistics from Altmetric.com
- DLB, dementia with Lewy bodies
- AD, Alzheimer's disease
- PD, Parkinson's disease
- SPET, single photon emission tomography
- FP-CIT, 2β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl)nortropane
- MMSE, mini mental state examination
- CAMCOG, Cambridge cognitive function examination
- CDR, clinical dementia rating
- BEHAVE-AD, behavioural pathology in Alzheimer's disease
- UPDRS, unified Parkinson's disease rating scale
- H&Y stage, Hoehn and Yahr stage
- CAPE, Clifton assessment procedure for the elderly
Dementia with Lewy bodies (DLB) is the second most common type of degenerative dementia after Alzheimer's disease (AD).1–3 The characteristic features of DLB are a progressive dementia with pronounced fluctuation in attention and levels of everyday activities, complex visual hallucinations, misperceptions, parkinsonism with frequent falls, and hypersensitivity to neuroleptic medication. As in AD, a definitive diagnosis can only be made by histopathological examination of the brain. A clinical diagnosis is made on the basis of probabilities by considering the symptoms, results of investigations and neuropsychological tests, and, in a research setting, according to agreed diagnostic criteria. However, a large proportion of patients with the clinical diagnosis of DLB also fulfil the NINCDS-ADRDA criteria4 for AD.
To improve diagnostic accuracy during life, in 1995 a consortium on dementia with Lewy bodies established “Consensus clinical and pathological criteria for DLB”.5 In a number of studies, the criteria were applied retrospectively to histopathologically confirmed cases of DLB. The specificity of the Consensus criteria has been reported to range from 29% to 100% and the sensitivity from 22% to 90%.6–12 Thus far there has only been one prospective study,13 in which the sensitivity of the Consensus criteria for DLB was 83% and the specificity was 95%. Thus, at best, the Consensus criteria perform fairly well, particularly in specialised centres with an interest in DLB, and certainly no worse than diagnostic criteria for AD14,15 or Parkinson's disease (PD),16 but nevertheless at least 15% of patients will continue to be misclassified.
There are a several reasons why it is important to separate DLB from other conditions during life. Patients with DLB warrant different management of their cognitive fluctuation, psychotic symptoms, and parkinsonian features.17 They have a worse prognosis if given neuroleptics, but respond well to cholinesterase inhibitors.18
The major neurochemical difference between AD and DLB is in the dopaminergic metabolism. Piggott et al19 studied postmortem brains from patients with PD, DLB, or AD, and elderly controls and showed that in DLB there is 72% reduction in the dopamine concentration in the putamen. This compares with 90% reduction in PD and no change in AD. In DLB, there is reduced binding to the dopamine uptake sites (presynaptic receptors) in the putamen (57%), but again this is not as pronounced as in PD (75%). There are no changes in uptake sites in AD compared with controls. The detection of this extensive dopaminergic degeneration would be expected to allow DLB to be distinguished from AD in vivo.
In DLB the loss of dopaminergic cells is accompanied by loss of the dopamine transporter (presynaptic receptors). This makes the dopamine transporter a surrogate marker for dopaminergic nigrostriatal neurones. Therefore, by imaging the dopamine transporter with single photon emission tomography (SPET), it is possible to assess the integrity of the nigrostriatal dopaminergic pathways.
Several ligands have been developed that have selective affinity for the dopamine transporter and can be used with SPET. The most promising SPET tracers for the dopamine transporter are cocaine analogues. A number of SPET studies in patients with PD have used the tracer 123I-labelled 2β-carbomethoxy-3β-(4-iodophenyl)tropane (β-CIT) to visualise the dopamine transporter.20–27 These studies showed a clear loss of striatal dopamine transporter in patients with PD compared with controls.
Important findings that bear on the usefulness of β-CIT in differentiating DLB from AD come from studies of patients with hemiparkinsonism. Marek et al28 showed that, in patients with hemiparkinsonism, striatal uptake was substantially reduced in the contralateral side (by 53%) but also in the ipsilateral side (by 38%). Similar results were obtained by Brucke et al.29 Thus a deficit in dopamine transporter, and therefore loss of dopaminergic neurones, can be shown with β-CIT at a time when no clinical signs are apparent. One would therefore expect that β-CIT SPET would detect the striatal dopaminergic deficit in patients with DLB. Donnemiller et al30 performed β-CIT SPET on seven patients with DLB, six patients with AD, and three controls. Uptake of radioactivity in the striatum was not significantly different in patients with AD and controls. Patients with DLB had significantly lower uptake than both controls and patients with AD. β-CIT does, however, have a significant drawback: its uptake in the human striatum is characterised by very slow kinetics. A stable level of striatal radioactivity (equilibrium of specific to non-specific uptake of radioactivity) is only achieved 20–30 hours after injection.31 This means that adequate images can only be acquired a day after injection of the ligand, and this diminishes its usefulness for outpatients.
Recently a new ligand, 123I-labelled 2β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl)nortropane (FP-CIT), has become available. As with β-CIT, in patients with PD there is bilateral reduction of striatal uptake of FP-CIT.32–34 FP-CIT is taken up more rapidly in the human striatum than β-CIT. The practical clinical advantage of the fast kinetics of FP-CIT is that patients can be scanned on the same day three to six hours after injection.
Our hypothesis was that patients with DLB (and those with PD) will have significantly lower uptake of FP-CIT (reflecting neuronal loss) than patients with AD and controls.
METHODS
Patients
Patients were recruited from the Derwent Memory Clinic, the old age psychiatry and neurology outpatient clinics, and hospital wards at Princess Alexandra Hospital, Harlow, St Margaret's Hospital, Epping, and the Whittington Hospital, London. Healthy elderly controls were recruited from relatives and partners of patients (mainly spouses). Controls were not taking any drugs known to affect the dopaminergic system. Dementia was diagnosed clinically. Patients were ascribed to either the DLB or the AD group by fulfilling the Consensus DLB criteria5 or NINCDS-ADRDA criteria.4 Many of the patients with DLB fulfilled both sets of criteria, and these patients were classified as having DLB. The diagnosis of PD was made according to the UK Parkinson's Disease Society Brain Bank criteria.16 Patients with PD were recruited from a neurology clinic at the time of first presentation to a neurologist (RWHW) and had not been exposed to any antiparkinsonian medication at the time of scanning. Neuropathological diagnosis was made as described by McKeith et al.13
Assessment
Patients with AD or DLB
For each patient a detailed history of memory impairment from the patient and an informant was obtained. This was followed by a full psychiatric history, a mental state examination, and a physical examination with an emphasis on neurological examination. A number of tests were performed:
the mini mental state examination (MMSE; adapted from Folstein et al35);
the Cambridge cognitive function examination (CAMCOG), a neuropsychological test battery which forms part of a standardised psychiatric assessment schedule, the Cambridge mental disorders of the elderly examination (CAMDEX)36;
the clinical dementia rating (CDR)37;
the Cornell scale for depression in dementia (Cornell depression scale)38;
the behavioural pathology in Alzheimer's disease rating scale (BEHAVE-AD)39;
the unified Parkinson's disease rating scale, motor part only (UPDRS)40;
the Hoehn and Yahr stage (H&Y stage)41;
the Clifton assessment procedure for the elderly, behavioural rating scale only (CAPE).42
Investigations
The following investigations were arranged for all patients if not already performed by a referring doctor: full blood count, erythrocyte sedimentation rate, urea and electrolytes, glucose, liver function tests, Ca2+, thyroid function test and syphilis serology, VDRL, vitamin B12, folate, and magnetic resonance imaging or computed tomography brain scan.
Patients with PD
Tests performed were: MMSE, CAMCOG, CDR, UPDRS, and H&Y stage.
Controls
Information was gathered from controls about their medical and psychiatric history and any regular medication. A mental state examination and a limited physical examination were carried out. Tests performed were: MMSE, CAMCOG, UPDRS, and H&Y stage.
FP-CIT SPET scan
SPET studies were carried out at the Institute of Nuclear Medicine, University College London Medical School. Thyroid metabolism was blocked with potassium iodate, as recommended, to reduce possible irradiation of the gland.
All subjects were scanned with a brain dedicated scanner, the Strichman Medical Equipment 810 linked to a Macintosh computer. The Strichman camera consists of 12 individual detectors, each equipped with a focusing collimator. The transaxial resolution of this camera is 7.6 mm full width half maximum, and axial resolution is 12.5 mm. The measured concentration of radioactivity was expressed as Strichman medical units (SMUs; 1 SMU=100 Bq/ml). Scanning took place between three and four hours after injection of FP-CIT (185 MBq). Usually 8–10 slices were acquired starting at the cerebellum level upwards to include basal ganglia. A few patients were difficult to scan, and, in these, a smaller number of slices (three to five) were acquired at the level of the basal ganglia to include the entire basal ganglion region. The overall scanning time for each patient was 30–45 minutes.
All scans were analysed by DCC, who was unaware of the diagnostic status of the subjects. The images were automatically reconstructed. For analysis of striatal binding, the ratio of specific to non-specific binding was calculated by summing up two to three adjacent transverse slices that showed the most intense striatal uptake. Regular circular regions of interest were used to calculate the average striatal (caudate nucleus, anterior and posterior putamen) to non-specific (areas with little or no dopamine receptors, such as occipital lobes) radioactivity ratios for both hemispheres (figs 1 and 2). The formula used was: where STR is the mean radioactivity (in SMUs) in the striatum (caudate, anterior and posterior putamen) and OCC is the mean radioactivity in the occipital cortex.
where STR is the mean radioactivity (in SMUs) in the striatum (caudate, anterior and posterior putamen) and OCC is the mean radioactivity in the occipital cortex.
FP-CIT scans of a healthy control (A) and patients with (B) Parkinson's disease, (C) Alzheimer's disease, or (D) dementia with Lewy bodies.
FP-CIT scan showing regions of interest in the caudate nucleus, anterior and posterior putamen, and occipital cortex.
Visual rating of scans
As a separate exercise, all the scans were presented randomly and assessed visually, purely qualitatively, by three independent raters (DCC, ZW, and RWHW) who were blind to the clinical and autopsy diagnoses. Scans were scored as follows: 0, normal uptake in all regions (right and left caudate and whole putamen); 1, slight reduction in uptake in any of the four regions; 2, significant reduction in uptake in any of the four regions. Subsequently for all correlations and statistical analyses, scans with scores of 0 or 1 were combined into a “normal” group and scans with a score of 2 were declared “abnormal”.
Statistical analysis
Data were analysed using SPSS/PC+ version 10.0 (Statistical Package for Social Sciences). FP-CIT binding was calculated for caudate, anterior and posterior putamen separately for each hemisphere. For patients with PD, “contralateral side” was defined as the side opposite to the clinically worse affected side. For patients with DLB or AD, it was difficult clinically to decide which side should be taken as the more affected, as some patients did not have any extrapyramidal signs, and therefore contralateral was arbitrarily assigned to the left side and ipsilateral to the right side. Analysis of variance and the t test were used to assess differences between the four groups (DLB, AD, PD, and controls) in ipsilateral and contralateral FP-CIT binding in the caudate and anterior and posterior putamen and between the four groups and their basic indices (age, MMSE, CAMCOG, CDR, BEHAVE-AD, UPDRS, Cornell depression scale, and CAPE). Cohen's κ test was used for inter-rater reliability.
Ethical approval
Ethical approval was obtained from West Essex Health Authority ethics committee, the Camden and Islington NHS Community Trust ethics committee, and the Administration of Radioactive Substances advisory committee of the United Kingdom.
RESULTS
As classified using clinical criteria, there were 27 patients with DLB, 17 patients with AD, 19 patients with PD, and 16 controls.
Basic characteristics of the cohort
Table 1 shows the frequency in patients with DLB or AD of: a family history of PD or AD; past psychiatric history; alcohol consumption; history of smoking; fluctuating course of illness, acute confusional states, visual hallucinations; tremor; rigidity; akinesia/bradykinesia. There was no significant difference between the two patient groups with respect to sex, the age at time of scan, age at onset of dementia, systolic blood pressure, and years of education (table 2).
Cohort characteristics of patients with dementia with Lewy bodies (DLB) or Alzheimer's disease (AD)
Cohort characteristics of patients with dementia with Lewy bodies (DLB), Alzheimer's disease (AD), or Parkinson's disease (PD), and controls
There were differences between patients with DLB and those with AD on the CDR, the MMSE, and the CAMCOG scores, the former being more severely demented. The Cornell depression scale scores were slightly higher for DLB than AD, but in neither group were they in the range indicating severe depression. Patients with DLB scored significantly worse (higher score) on CAPE and BEHAVE-AD than patients with AD (table 3).
Ratings of patients with dementia with Lewy bodies (DLB) or Alzheimer's disease (AD)
Patients with PD and controls were significantly younger than patients with DLB (p<0.01) and scored higher on the MMSE (MMSE mean DLB 16.2, AD 21.5 v PD 27.7, controls 28.9; p<0.001) and the CAMCOG (DLB 49.3, AD 69.8 v PD 98.8, controls 101.5; p<0.001).
As expected, both PD and DLB patients scored higher on the UPDRS and the H&Y stage than did patients with AD and controls (table 2; p<0.001).
Binding of FP-CIT radioactivity (semiquantitative method)
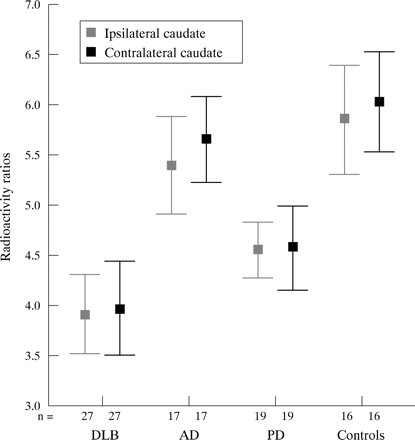
Table 4 and figs 3, 4, and 5 show the mean contralateral and ipsilateral radioactivity binding in the caudate nucleus and the anterior and posterior putamen in the four groups. Both the DLB and PD groups showed significantly lower uptake of radioactivity in all striatal areas than the AD group and controls (analysis of variance: p<0.001, in the contralateral and the ipsilateral caudate nucleus and anterior and posterior putamen). There were highly significant differences between DLB and AD, and DLB and controls for all ipsilateral and contralateral binding measures (t tests; p<0.001). There was a clear separation of the AD and DLB groups with no overlap of the 95% confidence intervals for the means of radioactivity binding. There were no significant differences in binding measures between patients with AD and controls in the ipsilateral and the contralateral caudate, the ipsilateral and the contralateral anterior putamen, and the ipsilateral posterior putamen, but patients with AD had lower binding than controls in the contralateral posterior putamen (p<0.05).
Semiquantitative FP-CIT binding measures
Radioactivity ratios in the caudate nucleus for patients with dementia with Lewy bodies (DLB), Alzheimer's disease (AD), or Parkinson's disease (PD) and healthy controls. Means and 95% confidence intervals are shown.
Radioactivity ratios in the anterior putamen for patients with dementia with Lewy bodies (DLB), Alzheimer's disease (AD), or Parkinson's disease (PD) and healthy controls. Means and 95% confidence intervals are shown.
Radioactivity ratios in the posterior putamen for patients with dementia with Lewy bodies (DLB), Alzheimer's disease (AD), or Parkinson's disease (PD) and healthy controls. Means and 95% confidence intervals are shown.
There were highly significant differences in all binding measures between PD and controls (p<0.001) and between PD and AD (p<0.005).
Simple qualitative visual assessment of scans
There was excellent agreement between the independent assessments of the specialist in nuclear medicine, the old age psychiatrist, and the neurologist (κ values 0.85, 0.89, 0.9). We compared the visual rating with the semiquantitative results by defining as abnormal any scan with contralateral posterior putamen binding that was more than 2 SDs below the mean of the controls (<3.02). The consensus visual rating (two or all three assessments in agreement) and the semiquantitative rating gave the same result (normal or abnormal scan) in 72/79 scans (91%), with κ 0.82, again an excellent agreement.
Autopsies
To date we have results of autopsies on 10 of the patients with dementia (table 5). These highlight shortcomings in the accuracy of the diagnoses made using the clinical diagnostic criteria, with an apparent tendency to overdiagnose DLB. The autopsy data have a number of effects on the results reported above. Reanalysing the data in the light of autopsy diagnoses where available, there are no longer significant differences between the DLB and AD groups with respect to: MMSE, CAMCOG, CDR, Cornell depression scale, BEHAVE-AD, and CAPE. The confidence intervals for the semiquantitative binding measures for the DLB group are all somewhat narrower, and importantly the separation of the DLB group from the AD group becomes greater (data not shown). Most importantly, with one exception (case 5), an abnormal scan always associates with an autopsy diagnosis of DLB, giving a sensitivity of 100% and a specificity of 83%, in comparison with high sensitivity but very low specificity for the clinical Consensus criteria for DLB in our clinics. In case 5, the explanation for the misleading scan is that the autopsy showed an infarct in the putamen on the side with low ligand binding, and in fact the binding values in the five other regions of that scan were well maintained, arguing in retrospect against a nigrostriatal degenerative process.
Comparison of clinical diagnosis, FP-CIT scan result, and autopsy in the 10 patients for whom autopsies have become available
DISCUSSION
This study shows that FP-CIT SPET clearly distinguishes a group of clinically classified patients with AD from a group of clinically classified patients with DLB. Furthermore, in this series, on the basis of final diagnoses made at autopsy, FP-CIT SPET scans performed better than clinical criteria in supporting the diagnosis of DLB in patients with dementia. The implication is that the use of FP-CIT SPET would improve the accuracy of diagnosis of DLB during life. Importantly, FP-CIT SPET could only be expected to support a clinical diagnosis, because any dementia that also involves nigrostriatal pathology, such as corticobasal degeneration or frontotemporal dementia with parkinsonism, or mixed pathology, may give rise to an abnormal FP-CIT SPET result, as indeed the case of putamen infarction in this study showed. Our patient with corticobasal degeneration had a scan that was “mildly abnormal” on visual inspection, but the posterior putamen binding was not in the abnormal range as we defined it.
This is the first study to show that it is possible to detect a clear reduction in striatal dopamine transporter in patients with DLB using FP-CIT SPET. It extends the observation by Donnemiller et al30 that patients with “diffuse Lewy body disease” have reduced striatal β-CIT uptake, and the observation by Hu et al43 using photon emission tomography that patients with DLB who have extrapyramidal signs and are responsive to L-dopa have significantly reduced uptake of 18F-flurodopa in the putamen and the caudate nuclei. Our findings agree with autopsy studies of DLB that show loss of neurones in the substantia nigra with concomitant reduction in dopamine reuptake sites and dopamine levels in the striatum.19,44
SPET scanning with FP-CIT appears to be clinically feasible. The scanning procedure was well tolerated by patients and controls. It was possible to obtain a good quality scan for all participants of the study, and no serious adverse effects were observed. The only possible adverse effect was that on one occasion a patient was incontinent during the scanning procedure.
The data show that simple visual rating of scans is effective, although we consider that the semiquantitative analysis of scans considerably enhances the information that can be obtained from them.
There were no significant differences in binding measures between patients with AD and controls apart from in the contralateral posterior putamen, where patients with AD had 10% lower counts than controls. Lavalaye et al45 have shown that the density of dopamine transporter declines with age, with a decrease in FP-CIT binding in the striatum of about 4% per decade. The healthy controls were about 10 years younger than the patients with AD and DLB, and the age difference may explain the slightly lower counts in patients with AD. It is also possible that some of the patients assigned clinically to the AD group actually have DLB.
Although other groups have reported good sensitivity and specificity for the Consensus criteria for DLB,6,11–13 their diagnostic accuracy in the present study has so far been disappointing; the reason for the lower specificity is not clear. It may simply be a chance phenomenon, and the numbers of cases are small (the specificity was 100% in a previous small series from our group46). The fact that all cases that fulfilled both AD and DLB diagnostic criteria were classified as DLB is also relevant. Nevertheless, the study reported here suggests that the incorporation of the results of functional imaging of the striatum into diagnostic criteria would greatly improve the precision of diagnosis of DLB during life.
Acknowledgments
We are grateful to the patients and their relatives who took part in the study. We also thank Mr Kenneth Connolly from the pathology department at Princess Alexandra Hospital and Dr Evelyn Jaros, Professor Robert Perry, and Ms Jean Dawes from the MRC Neuropathology Unit in Newcastle. The work was supported by grants from Nycomed Amersham plc, UK and Novartis.
REFERENCES
Footnotes
-
Competing interests: DCC received some financial support from Nycomed Amersham plc, now called Amersham Healthcare. IGMcK has received consultancy fees and research funds from Nycomed Amersham plc. ZW has received research funds from Nycomed Amersham plc.