Published online Aug 26, 2014. doi: 10.4330/wjc.v6.i8.782
Revised: March 29, 2014
Accepted: May 31, 2014
Published online: August 26, 2014
Chagas disease cardiomyopathy (CCC), the main consequence of Trypanosoma cruzi (T.cruzi) infection, is an inflammatory cardiomyopathy that develops in up to 30% of infected individuals. The heart inflammation in CCC patients is characterized by a Th1 T cell-rich myocarditis with increased production of interferon (IFN)-γ, produced by the CCC myocardial infiltrate and detected at high levels in the periphery. IFN-γ has a central role in the cardiomyocyte signaling during both acute and chronic phases of T.cruzi infection. In this review, we have chosen to focus in its pleiotropic mode of action during CCC, which may ultimately be the strongest driver towards pathological remodeling and heart failure. We describe here the antiparasitic protective and pathogenic dual role of IFN-γ in Chagas disease.
Core tip: Chagas disease cardiomyopathy (CCC) occurs in 30% of those infected with the protozoan Trypanosoma cruzi, endemic in Latin America. It is an inflammatory cardiomyopathy with a worse prognosis than cardiomyopathies of other etiologies. Interferon (IFN)- γ is the main cytokine produced locally and induces strong signaling in cardiomyocytes. This review focuses on the pleiotropic protective and pathogenic effects of IFN-γ on CCC.
- Citation: Ferreira LRP, Frade AF, Baron MA, Navarro IC, Kalil J, Chevillard C, Cunha-Neto E. Interferon-γ and other inflammatory mediators in cardiomyocyte signaling during Chagas disease cardiomyopathy. World J Cardiol 2014; 6(8): 782-790
- URL: https://www.wjgnet.com/1949-8462/full/v6/i8/782.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i8.782
Chagas disease cardiomyopathy (CCC) is a particularly aggressive inflammatory dilated cardiomyopathy that occurs decades after the initial infection with the obligate intracellular parasite Trypanosoma cruzi (T.cruzi) in 30% of infected individuals[1]. T. cruzi infection affects 10 million subjects in endemic areas of South and Central America and migratory waves have taken patients to the United States, Europe and Japan[2-4]. Patients with CCC have a worse clinical progression and survival than those with cardiomyopathy of other etiologies. The development of CCC is associated with inflammation and activation of the immune system, with a local increased cardiac production of cytokines by the heart-infiltrating T cells and other mononuclear cells[5]. These mononuclear cells infiltrating CCC heart tissue express predominantly interferon (IFN)-γ and tumor necrosis factor (TNF)-α, with lower levels of interleukin (IL)-2, IL-4, IL-6 and IL-10. Cytokines like IL-7 and IL-15, which promote T cell survival, are also found to have increased expression in CCC heart tissue[6,7]. Significant IFN-γ signaling was observed in the myocardium of CCC patients, including genes that are not ordinarily expressed by inflammatory cells[8]. A similar increase in IFN-γ and TNF-α expression is observed in cardiac tissue from animals infected with T. cruzi[9]. CCC patients have a progressive myocardial remodeling process with hypertrophy and fibrosis causing heart fiber damage, heart conduction abnormalities, arrhythmias, apical aneurysm, heart failure and sudden death[10,11]. Several hypotheses have been raised to explain the lesions in the myocardium of CCC, which includes persistence of the parasite or its antigens at the inflammatory site and autoimmune tissue damage[5,12]. There are two drugs available to treat the acute phase of the disease, nifurtimox (nitrofurane) and benznidazole (nitroimidazole). The use of these drugs to treat the acute phase of the disease is widely accepted. However, their use in the treatment of the chronic phase is controversial. There is no specific treatment, against the parasite, that can benefit patients at the chronic stage of Chagas disease[13]. The undesirable side effects of both drugs are a major drawback in their use, frequently forcing the physician to stop treatment. The treatment of chronic patients consists of control of the symptoms and improvement in quality of life, by preventing cardiovascular complications according to the guidelines for treating heart failure and arrhythmias[14]. Regardless of the mechanisms underlying the initiation and maintenance of the myocarditis, the bulk of the evidence indicates that the inflammatory infiltrate is a significant effector of heart tissue damage. Our group has demonstrated over the past several years that, aside from direct inflammatory damage, several cytokines and chemokines produced in the myocardium of CCC patients may also have a non-immunological pathogenic effect beyond direct inflammatory tissue damage, via modulation of gene and protein expression in cardiomyocytes and other myocardial cell types[5,7,15,16]. While IFN-γ acts as an immunological mediator during the acute stage of the disease suppressing overt parasitism, in the chronic phase of the disease it will both curtail parasitism and cause tissue damage through immunological and non-immunological effects entertaining the gradual progression to CCC.
IFN-γ is a protein with 146 amino acid residues, the only member of the type II IFN family, and in humans is encoded by a chromosomal locus separate from type I IFNs, on chromosome 12q24.1 with approximately 5.4 kb and four exons[17]. IFN-γ is mainly produced by CD4+ T helper cell type 1 (Th1) lymphocytes, CD8+ cytotoxic lymphocytes, and natural killer (NK) cells, but can also be produced by other cells, such as B cells, NKT cells, and professional antigen-presenting cells (APCs). Cytokines secreted by APCs, most notably IL-12 and IL-18, control IFN-γ production and differentiation of cells capable of producing the cytokine. Interaction of macrophages and other APCs with pathogen-associated molecular patterns (PAMPs) induces secretion of IL-12 and chemokines. These chemokines attract inflammatory cells to the site of inflammation, and IL-12 promotes IFN-γ synthesis in these cells[18]. Negative regulators of IFN-γ production include IL-4, IL-10, transforming growth factor (TGF)-β, and glucocorticoids[19]. Animal models as well the analysis of different human diseases are good examples of the paradoxical roles of IFN-γ. Mice lacking IFN-γ and its receptor (IFNGR) showed no developmental defects, and their immune system appeared to develop normally[20]. However, these mice show deficiencies in natural resistance to infection. In humans, inactivating mutations of the human IFNGR1 or IFNGR2 chains show clinical presentation similar to the mouse models. At the same time IFN-γ can be beneficial in infectious diseases where it strengthens cellular defense mechanisms and favors the generation of specific immunity, and can be disease-promoting as described in non-infectious diseases. Reifenberg et al[21] have shown that SAP-IFN-γ transgenic mice, which constitutively express IFN-γ in their livers, developed chronic active myocarditis. These mice exhibited IFN-γ-mediated cardiotoxicity with left ventricular dilation and impaired systolic function, a true cardiomyopathy[21]. Morino et al[22] have reported a case of cardiomyopathy in a renal cell carcinoma patient treated with IFN-γ. In humans, IFN-γ is also implicated in the pathology of diseases such as systemic lupus erythematous[23], insulin-dependent diabetes mellitus[24] and multiple sclerosis[25]. Like other cytokines, the IFN-γ coding region is invariant with no reported polymorphisms. However, single nucleotide polymorphisms (SNPs) in intronic regions have been described and a microsatellite polymorphism consisting of a dinucleotide (CA) repeat in the first intron is the one most extensively studied as it is correlated with high IFN-γ production[26]. An association between IFN-γ SNPs and diseases like rheumatoid arthritis has been reported[27,28]. Nevertheless, as a cytokine with ambiguous effects, IFN-γ polymorphisms are correlated with increased longevity[25]. It has been proposed that a slightly dampened inflammatory status caused by an IFN-γ polymorphism, while not enough to significantly impact on the individual’s ability to clear infection, may prevent or defer inflammation-related diseases such as cardiovascular disease, neurodegeneration, osteoarthritis, osteoporosis, and diabetes[29]. In experimental T. cruzi infection, it has been shown by several investigators that IFN-γ can enhance macrophage killing of the parasite in vitro and increase resistance to an infectious challenge in vivo, an effect dependent on the de novo synthesis of TNF-α and NO by infected macrophages[9,30,31]. It has also been demonstrated that parasite-induced IFN-γ produced during T. cruzi infection by T and NK cells is involved in resistance to infection and protection in mice. This protection seems to be dependent on the IFN-γ/TNF-α pathway[31].
Although infective T. cruzi trypomastigotes are capable of invading a wide variety of tissues and cell types in the vertebrate host, the majority of T.cruzi laboratory strains and isolates have tropism for cardiac tissue and or cardiomyocytes[32]. The establishment of a long-term infection in the heart and the development of a cardiomyopathy condition are directly related to the ability of T. cruzi to infect and persist within cardiomyocytes during the acute phase of infection[33,34]. Cardiomyocytes are differentiated cells that respond to T.cruzi infection by initiating adaptive strategies. These strategies can involve immunological and non-immunological events. For example, during T.cruzi infection cardiomyocytes reactivate an embryonic gene expression pattern[8] (e.g., an increase in expression of atrial natriuretic factor), inhibit apoptosis[34], increase cell size by producing myofibrils (cardiac myosin heavy chain, several α-actin isoforms, smooth muscle myosin, actin-binding proteins, and collagen) and initiate a hypertrophic program, that are not related to an immunological response to the parasite[7]. However, these cells are actively integrated in the inflammatory process and can secrete chemokines such as C-C chemokine monocyte chemotactic protein 1 (JE/MCP-1/CCL2), chemokine (C-C motif) ligand 5 (RANTES/CCL5), keratinocyte chemoattractant (KC/CXCL3), macrophage inflammatory protein (MIP-2/CXCL2), Mig/CXCL9, and cytokine-responsive gene-2 (Crg-2/CXCL10), and the cytokines TNF-α, IL-1β and inducible NO synthase (iNOS)[35]. These chemokines will drive the early influx of leukocytes, and influence T-helper cell recruitment and local IFN-γ production defining the inflammatory infiltrate in the hearts during experimental T.cruzi infection and, presumably, also in acutely infected patients. It was recently demonstrated that there is a segregation of CD8+ cell populations in the heart in T.cruzi infected mice into two groups: CD8+ T cells producing perforin and no IFN-γ (IFN-γneg Pfn+) and perforin-negative and IFN-γ-producing cells (IFN-γ+ Pfnneg). These data supported the idea that CD8+ Pfn+ T-cells are involved in cardiomyocyte injury during T. cruzi infection, whereas CD8+ IFN-γ+ cells play a beneficial role in cardiomyocyte damage[36].
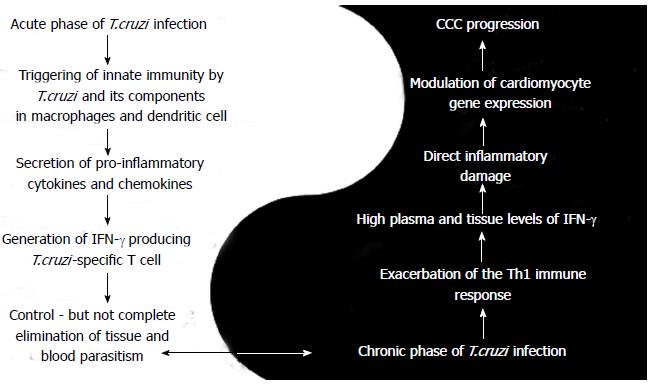
A dual role in pathogenesis and protection during Chagas disease was described for IFN-γ and other cytokines, such as TNF-α[37]. Bahia-Oliveira et al[38], taking into account only the inflammatory actions of the cytokine, also described the dual role of IFN-γ during chronic Chagas disease. Our observations from the standpoint of the pleiotropic biological effects, both inflammatory and non-inflammatory, in Chagas disease made us remodel the concept as will follow in these review. During T. cruzi infection, once the inflammatory process starts, IFN-γ will be produced by Th1 cells and act as a prime inflammatory cytokine in different pathways of the immune system, such as upregulating MHC class I and classII molecules, suppressing Th2 immune responses by antagonism of IL-4 production, inducing high levels of antigen presentation and activating macrophages[18]. Our group has demonstrated the importance of IFN-γ, TNF-α and several chemokines in CCC by showing that they play a role in the generation of the inflammatory infiltrate[8,15,39]. CCC patients have an increased peripheral production of IFN-γ and TNF-α when compared to patients with the asymptomatic/indeterminate form. On the other hand, IFN-γ has direct effects on cardiomyocytes and perhaps other cells of the myocardium[8]. In the following sections we describe in detail the dual mechanism of IFN-γ during Chagas disease (acute and chronic phases) as illustrated in Figure 1.
Data from animal models and from the earliest stages in a proportion of naturally infected individuals has shown that inflammatory cytokines such as IFN-γ play a central role in acute T. cruzi infection. During invasion, T. cruzi or its derived molecules like DNA and glycosylphosphatidylinositol-anchored mucin-like glycoproteins derived from trypomastigotes forms (tGPI-mucins) can stimulate the host cutaneous cells, macrophages, cardiomyocytes and dendritic cells (as seen in in vivo and in vitro infection) to produce mediators that will trigger a local inflammatory response[40]. This activation will induce these cells to promptly release pro-inflammatory cytokines such as IL-1, IL-6, IL-12, IL-18, IL-27 and TNF-α and further activate other inflammatory cells. These cytokines will participate in the control of the infection, killing the parasite with the help of NO production via iNOS/NOS2. T. cruzi-specific T cells will produce IFN-γ, which in conjunction with macrophages producing TNF-α will migrate with other blood leukocytes to the site of inflammation in response to chemokines such as CCL2, CCL3, CCL4, CCL5, CXCL10 and CCR5[41]. The blockade of one, CCR5, by Met-RANTES significantly decreased the intensity of cardiac inflammatory infiltrate, suggesting that lymphocyte migration to the myocardium during acute infection is dependent on CCR5 ligands[42,43]. IFN-γ-inducible adhesion molecules, such as fibronectin and VCAM-1, can also be detected at high levels in cardiac tissue from T. cruzi-infected mice[44]. Few studies have investigated the immunology of the acute phase infection in human patients. It has been described that acutely infected children display increased expression of inflammatory cytokines, such as circulating IL-6 and TNF-α[45] and increased production of IFN-γ by mononuclear cells[46]. Serum C-reactive protein (CRP) and IL-6 concentrations have also been shown to increase in children infected with T. cruzi during the acute phase, but not in the chronic phase of Chagas disease[47].
During the chronic phase of T. cruzi infection, CCC patients have an exacerbation of the Th1 immune response compared with those with the indeterminate form of Chagas disease. It was observed that CCC patients displayed greater cytokine production (Table 1) by mononuclear cells, higher plasma levels of TNF-α and IFN-γ and an increased number of IFN-γ-producing CCR5+CXCR3+CD4+ and CD8+ T cells, with reduced numbers of IL-10-producing and FoxP3+ regulatory T cells[15,48-50]. It has been hypothesized that this increased production of IL-10 by regulatory T cells restricts Th1 T cell differentiation and IFN-γ production in the majority of chronically T. cruzi-infected individuals, leading to the asymptomatic form of the disease[15,48-50]. Aside from the delayed hypersensitivity type of tissue damage classically seen in tissue lesions induced by IFN-γ, with cardiomyocyte loss and fibrosis, the local production of IFN-γ in CCC heart lesions can induce profound changes in the cardiomyocyte gene expression pattern as observed by our group using cDNA microarrays. Significant IFN-γ signaling was observed in the myocardium of CCC patients, including genes that are not ordinarily expressed by inflammatory cells. We have observed that 15% of the genes selectively upregulated in CCC are IFN-γ-inducible genes, including inflammatory response genes expressed by the infiltrating inflammatory cells (e.g., cytokine receptors, immunoglobulin, T cell receptor genes). Several IFN-γ modulated genes are not expressed by inflammatory cells, including angiotensin II receptor 2, fatty acid-binding protein 5, cardiovascular 27-kd Hsp and genes encoding a number of proteins involved in oxidative phosphorylation and lipid catabolism in the CCC myocardium, compared with idiopathic dilated cardiomyopathy or donor myocardium[8]. cDNA microarray experiments in mice infected with T. cruzi showed changes in oxidative phosphorylation and depressed energy metabolism[51] and respiratory chain complexes with a reduced ATP-generating capacity[52]. Moreover, expression profiling in hearts of mice infected by T. cruzi also showed diminished myocardial energy metabolism and altered oxidative phosphorylation[51,53]. Significantly, mice infected for 100 d showed morphological alterations in the mitochondria and diminished expression of genes from the oxidative phosphorylation pathway, with a detectable reduction in OXPHOS-mediated mitochondrial ATP production[51]. Thus, both IFN-γ and T. cruzi infection can depress energy metabolism to reduce myocardial ATP generation, which has potential consequences for myocardial contractility, electric conduction and rhythm. Interestingly, one of the genes downregulated in CCC hearts, SERCA Ca2+-ATPase is repressible by IFN-γ and is also involved in cardiac metabolism. In vitro experiments have shown that IFN-γ may induce profound changes in the cardiomyocyte gene expression program, including induction of atrial natriuretic factor and of the hypertrophic gene expression program, which can ultimately lead to heart dilation and heart failure[8]. Other inflammatory mediators and chemokines such as IL-18 and CCR7 ligands, upregulated in the CCC myocardium[39], induce cardiomyocyte hypertrophy and molecules involved in the fibrotic process[54-56]. Transgenic mice overexpressing CCL2, TNF-α or IFN-γ in the myocardium develop myocardial hypertrophy and ventricular dilation[21,57,58]. Inflammatory cytokines may also affect myocardial energy metabolism, and ventricular dysfunction is associated with reduced energy metabolism[59,60]. Treatment of cardiomyocytes with IFN-γ inhibited oxidative metabolism and ATP production[61] and reduced gene and protein expression of creatine kinase, which is responsible for translocation of mitochondrial ATP to the sarcoplasm in cultured human skeletal muscle cells[62]. We observed that the myocardium of CCC patients displays reduced expression of some key energy metabolism enzymes, including isoforms of creatine kinases, Krebs cycle enzymes, and members of the ATP synthase complex, in comparison with the myocardium of patients with non-inflammatory cardiomyopathies and heart donors (unpublished observations), which could be partly due to IFN-γ inflammatory cytokine signaling. cDNA Microarray experiments in mice experimentally infected with T. cruzi showed changes in oxidative phosphorylation and depressed energy metabolism[51], and respiratory chain complexes with a reduced ATP-generating capacity[52]. Thus, both IFN-γ and T. cruzi infection can depress energy metabolism, reducing myocardial ATP generation, with potential consequences for myocardial contractility, electrical conduction and rhythm. Taken together, data show that, apart from the direct inflammatory damage, the non-immunological effects of IFN-γ in the myocardium may play a significant pathogenic role in CCC, resulting in disease progression observed by a high degree of heart failure-inducing hypertrophy and fibrosis. The in-depth understanding of these pathways may lead to the development of new therapies for CCC.
| Cytokines/chemokines | Phase (acute /chronic/IND /severe/moderate CCC) | Host (mouse/human) | Organ/cell type | Ref. |
| IFN-γ | Severe CCC | Human | Mononuclear cells | [15,49] |
| IFN-γ | Severe CCC | Human | Myocardium | [63,64] |
| IFN-γ | Severe CCC | Human | Heart-infiltrating T cells | [15] |
| IFN-γ | IND, Severe CCC | Human | Plasma | [15,65,66] |
| TNF-α | Severe CCC | Human | Mononuclear cells | [15,49] |
| TNF-α | Severe CCC | Human | Heart-infiltrating T cells | [15] |
| TNF-α | Severe CCC | Human | Myocardium | [63,64] |
| TNF-α | IND and Severe CCC | Human | plasma | [15,65,66] |
| IFN-γ | Acute/chronic | Mouse | Heart | [67-69] |
| TNF-α | Acute/chronic | Mouse | Heart | [70] |
| IL-6 | Severe CCC | Human | Heart-infiltrating T cells | [15,63,64] |
| IL-2 | Severe CCC | Human | Heart-infiltrating T cells | [15,63,64] |
| IL-4 | Severe CCC | Human | Heart-infiltrating T cells | [15,63,64] |
| IL-10 | Severe CCC | Human | Heart-infiltrating T cells | [15, 63, 64] |
| IL-7 | Severe CCC | Human | Myocardium | [71] |
| IL-15 | Severe CCC | Human | Myocardium | [71] |
| IL-12 | Acute | Mouse | Mononuclear cells | [72] |
| IL-18 | Acute | Mouse | Mononuclear cells | [73] |
| IL-10 | Acute | Mouse | Mononuclear cells | [74-76] |
| TGF-β | Acute | Mouse | Mononuclear cells | [74-76] |
| IL-17 | Chronic | Mouse | Mononuclear cells | [77] |
| CCL2, CXCL10, CXCL9 (mRNA) | Severe CCC | Human | Myocardium | [8] |
| CCR2, CXCR3 (mRNA) | Severe CCC | Human | Myocardium | [8] |
| CCR5, CXCR3 | Severe CCC, IND | Human | Mononuclear cells | [48] |
| CCL5, CXCL9, CXCL10 | Chronic | Mouse | Cardiomyocytes | [35] |
| CCR5 | Chronic | Mouse | Heart | [43,78] |
| CCL5, CCL4, CXCR3 (mRNA) | Chronic | Dog | Heart | [79] |
Authors received financial assistance from CNPq (Brazilian National Research Council), FAPESP (São Paulo State Research Funding Agency-Brazil) and Institut National de la Santé et de la Recherche Médicale (INSERM), the Aix-Marseille University (Direction des Relations Internationales), USP-COFECUB program, the ARCUS II PACA Brésil program. LRPF is recipient of Brazilian Council for Scientific and Technological Development - CNPq fellowship. AFF, MAB, ICN are recipients of a São Paulo State Research Funding Agency - FAPESP fellowship. ECN and CC were recipients for an international program funded either by the French ANR (Br-Fr-CHAGAS) and the Brazilian FAPESP agencies. CC is a recipient of a temporary professor position supported by the French consulate in Brazil and the University of São Paulo.
P- Reviewer: Al-Biltagi M, Ciampi Q, Fett JD, Xiong XJ S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
| 1. | Frade AF, Pissetti CW, Ianni BM, Saba B, Lin-Wang HT, Nogueira LG, de Melo Borges A, Buck P, Dias F, Baron M. Genetic susceptibility to Chagas disease cardiomyopathy: involvement of several genes of the innate immunity and chemokine-dependent migration pathways. BMC Infect Dis. 2013;13:587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Kapelusznik L, Varela D, Montgomery SP, Shah AN, Steurer FJ, Rubinstein D, Caplivski D, Pinney SP, Turker D, Factor SH. Chagas disease in Latin American immigrants with dilated cardiomyopathy in New York City. Clin Infect Dis. 2013;57:e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Ventura-Garcia L, Roura M, Pell C, Posada E, Gascón J, Aldasoro E, Muñoz J, Pool R. Socio-cultural aspects of Chagas disease: a systematic review of qualitative research. PLoS Negl Trop Dis. 2013;7:e2410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Gascon J, Bern C, Pinazo MJ. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 2010;115:22-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 418] [Cited by in F6Publishing: 410] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 5. | Cunha-Neto E, Teixeira PC, Fonseca SG, Bilate AM, Kalil J. Myocardial gene and protein expression profiles after autoimmune injury in Chagas’ disease cardiomyopathy. Autoimmun Rev. 2011;10:163-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Machado FS, Dutra WO, Esper L, Gollob KJ, Teixeira MM, Factor SM, Weiss LM, Nagajyothi F, Tanowitz HB, Garg NJ. Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease. Semin Immunopathol. 2012;34:753-770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 7. | Cunha-Neto E, Nogueira LG, Teixeira PC, Ramasawmy R, Drigo SA, Goldberg AC, Fonseca SG, Bilate AM, Kalil J. Immunological and non-immunological effects of cytokines and chemokines in the pathogenesis of chronic Chagas disease cardiomyopathy. Mem Inst Oswaldo Cruz. 2009;104 Suppl 1:252-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Cunha-Neto E, Dzau VJ, Allen PD, Stamatiou D, Benvenutti L, Higuchi ML, Koyama NS, Silva JS, Kalil J, Liew CC. Cardiac gene expression profiling provides evidence for cytokinopathy as a molecular mechanism in Chagas’ disease cardiomyopathy. Am J Pathol. 2005;167:305-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Aliberti JC, Souto JT, Marino AP, Lannes-Vieira J, Teixeira MM, Farber J, Gazzinelli RT, Silva JS. Modulation of chemokine production and inflammatory responses in interferon-gamma- and tumor necrosis factor-R1-deficient mice during Trypanosoma cruzi infection. Am J Pathol. 2001;158:1433-1440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Mady C, Cardoso RH, Barretto AC, da Luz PL, Bellotti G, Pileggi F. Survival and predictors of survival in patients with congestive heart failure due to Chagas’ cardiomyopathy. Circulation. 1994;90:3098-3102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 120] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Pimentel Wde S, Ramires FJ, Lanni BM, Salemi VM, Bilate AM, Cunha-Neto E, Oliveira AM, Fernandes F, Mady C. The effect of beta-blockade on myocardial remodelling in Chagas’ cardiomyopathy. Clinics (Sao Paulo). 2012;67:1063-1069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Gutierrez FR, Guedes PM, Gazzinelli RT, Silva JS. The role of parasite persistence in pathogenesis of Chagas heart disease. Parasite Immunol. 2009;31:673-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. | Diniz Lde F, Urbina JA, de Andrade IM, Mazzeti AL, Martins TA, Caldas IS, Talvani A, Ribeiro I, Bahia MT. Benznidazole and posaconazole in experimental Chagas disease: positive interaction in concomitant and sequential treatments. PLoS Negl Trop Dis. 2013;7:e2367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 14. | Pinazo MJ, Thomas MC, Bua J, Perrone A, Schijman AG, Viotti RJ, Ramsey JM, Ribeiro I, Sosa-Estani S, López MC. Biological markers for evaluating therapeutic efficacy in Chagas disease, a systematic review. Expert Rev Anti Infect Ther. 2014;12:479-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Abel LC, Rizzo LV, Ianni B, Albuquerque F, Bacal F, Carrara D, Bocchi EA, Teixeira HC, Mady C, Kalil J. Chronic Chagas’ disease cardiomyopathy patients display an increased IFN-gamma response to Trypanosoma cruzi infection. J Autoimmun. 2001;17:99-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 144] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Bilate AM, Cunha-Neto E. Chagas disease cardiomyopathy: current concepts of an old disease. Rev Inst Med Trop Sao Paulo. 2008;50:67-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Bream JH, Ping A, Zhang X, Winkler C, Young HA. A single nucleotide polymorphism in the proximal IFN-gamma promoter alters control of gene transcription. Genes Immun. 2002;3:165-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2690] [Cited by in F6Publishing: 2850] [Article Influence: 135.7] [Reference Citation Analysis (0)] |
| 19. | Schindler H, Lutz MB, Röllinghoff M, Bogdan C. The production of IFN-gamma by IL-12/IL-18-activated macrophages requires STAT4 signaling and is inhibited by IL-4. J Immunol. 2001;166:3075-3082. [PubMed] [Cited in This Article: ] |
| 20. | Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel RM, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742-1745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1238] [Cited by in F6Publishing: 1236] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 21. | Reifenberg K, Lehr HA, Torzewski M, Steige G, Wiese E, Küpper I, Becker C, Ott S, Nusser P, Yamamura K. Interferon-gamma induces chronic active myocarditis and cardiomyopathy in transgenic mice. Am J Pathol. 2007;171:463-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Morino Y, Hara K, Ushikoshi H, Tanabe K, Kuroda Y, Noguchi T, Ayabe S, Hara H, Yanbe Y, Kozuma K. Gamma-interferon-induced cardiomyopathy during treatment of renal cell carcinoma: a case report. J Cardiol. 2000;36:49-57. [PubMed] [Cited in This Article: ] |
| 23. | Lee JY, Goldman D, Piliero LM, Petri M, Sullivan KE. Interferon-gamma polymorphisms in systemic lupus erythematosus. Genes Immun. 2001;2:254-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Yi Z, Li L, Garland A, He Q, Wang H, Katz JD, Tisch R, Wang B. IFN-γ receptor deficiency prevents diabetes induction by diabetogenic CD4+, but not CD8+, T cells. Eur J Immunol. 2012;42:2010-2018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Panitch HS, Hirsch RL, Haley AS, Johnson KP. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987;1:893-895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 547] [Cited by in F6Publishing: 509] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 26. | Macmurray J, Comings DE, Napolioni V. The gene-immune-behavioral pathway: Gamma-interferon (IFN-γ) simultaneously coordinates susceptibility to infectious disease and harm avoidance behaviors. Brain Behav Immun. 2013;Sep 25; Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 27. | Khani-Hanjani A, Lacaille D, Hoar D, Chalmers A, Horsman D, Anderson M, Balshaw R, Keown PA. Association between dinucleotide repeat in non-coding region of interferon-gamma gene and susceptibility to, and severity of, rheumatoid arthritis. Lancet. 2000;356:820-825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Lio D, Balistreri CR, Colonna-Romano G, Motta M, Franceschi C, Malaguarnera M, Candore G, Caruso C. Association between the MHC class I gene HFE polymorphisms and longevity: a study in Sicilian population. Genes Immun. 2002;3:20-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Lio D, Marino V, Serauto A, Gioia V, Scola L, Crivello A, Forte GI, Colonna-Romano G, Candore G, Caruso C. Genotype frequencies of the +874T--& gt; A single nucleotide polymorphism in the first intron of the interferon-gamma gene in a sample of Sicilian patients affected by tuberculosis. Eur J Immunogenet. 2002;29:371-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Talvani A, Ribeiro CS, Aliberti JC, Michailowsky V, Santos PV, Murta SM, Romanha AJ, Almeida IC, Farber J, Lannes-Vieira J. Kinetics of cytokine gene expression in experimental chagasic cardiomyopathy: tissue parasitism and endogenous IFN-gamma as important determinants of chemokine mRNA expression during infection with Trypanosoma cruzi. Microbes Infect. 2000;2:851-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 153] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Rodrigues AA, Saosa JS, da Silva GK, Martins FA, da Silva AA, Souza Neto CP, Horta CV, Zamboni DS, da Silva JS, Ferro EA. IFN-γ plays a unique role in protection against low virulent Trypanosoma cruzi strain. PLoS Negl Trop Dis. 2012;6:e1598. [PubMed] [Cited in This Article: ] |
| 32. | Andrade LO, Galvão LM, Meirelles Mde N, Chiari E, Pena SD, Macedo AM. Differential tissue tropism of Trypanosoma cruzi strains: an in vitro study. Mem Inst Oswaldo Cruz. 2010;105:834-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Nagajyothi F, Machado FS, Burleigh BA, Jelicks LA, Scherer PE, Mukherjee S, Lisanti MP, Weiss LM, Garg NJ, Tanowitz HB. Mechanisms of Trypanosoma cruzi persistence in Chagas disease. Cell Microbiol. 2012;14:634-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 34. | Petersen CA, Krumholz KA, Carmen J, Sinai AP, Burleigh BA. Trypanosoma cruzi infection and nuclear factor kappa B activation prevent apoptosis in cardiac cells. Infect Immun. 2006;74:1580-1587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Machado FS, Martins GA, Aliberti JC, Mestriner FL, Cunha FQ, Silva JS. Trypanosoma cruzi-infected cardiomyocytes produce chemokines and cytokines that trigger potent nitric oxide-dependent trypanocidal activity. Circulation. 2000;102:3003-3008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 189] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 36. | Silverio JC, Pereira IR, Cipitelli Mda C, Vinagre NF, Rodrigues MM, Gazzinelli RT, Lannes-Vieira J. CD8+ T-cells expressing interferon gamma or perforin play antagonistic roles in heart injury in experimental Trypanosoma cruzi-elicited cardiomyopathy. PLoS Pathog. 2012;8:e1002645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Lannes-Vieira J, Pereira IR, Vinagre NF, Arnez LE. TNF-α and TNFR in Chagas disease: from protective immunity to pathogenesis of chronic cardiomyopathy. Adv Exp Med Biol. 2011;691:221-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Bahia-Oliveira LM, Gomes JA, Rocha MO, Moreira MC, Lemos EM, Luz ZM, Pereira ME, Coffman RL, Dias JC, Cançado JR. IFN-gamma in human Chagas’ disease: protection or pathology? Braz J Med Biol Res. 1998;31:127-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Nogueira LG, Santos RH, Ianni BM, Fiorelli AI, Mairena EC, Benvenuti LA, Frade A, Donadi E, Dias F, Saba B. Myocardial chemokine expression and intensity of myocarditis in Chagas cardiomyopathy are controlled by polymorphisms in CXCL9 and CXCL10. PLoS Negl Trop Dis. 2012;6:e1867. [PubMed] [Cited in This Article: ] |
| 40. | Almeida IC, Gazzinelli RT. Proinflammatory activity of glycosylphosphatidylinositol anchors derived from Trypanosoma cruzi: structural and functional analyses. J Leukoc Biol. 2001;70:467-477. [PubMed] [Cited in This Article: ] |
| 41. | Kroll-Palhares K, Silvério JC, Silva AA, Michailowsky V, Marino AP, Silva NM, Carvalho CM, Pinto LM, Gazzinelli RT, Lannes-Vieira J. TNF/TNFR1 signaling up-regulates CCR5 expression by CD8+ T lymphocytes and promotes heart tissue damage during Trypanosoma cruzi infection: beneficial effects of TNF-alpha blockade. Mem Inst Oswaldo Cruz. 2008;103:375-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Marino AP, Silva AA, Santos PV, Pinto LM, Gazinelli RT, Teixeira MM, Lannes-Vieira J. CC-chemokine receptors: a potential therapeutic target for Trypanosoma cruzi-elicited myocarditis. Mem Inst Oswaldo Cruz. 2005;100 Suppl 1:93-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Marino AP, da Silva A, dos Santos P, Pinto LM, Gazzinelli RT, Teixeira MM, Lannes-Vieira J. Regulated on activation, normal T cell expressed and secreted (RANTES) antagonist (Met-RANTES) controls the early phase of Trypanosoma cruzi-elicited myocarditis. Circulation. 2004;110:1443-1449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Marino AP, Azevedo MI, Lannes-Vieira J. Differential expression of adhesion moleculesshaping the T-cell subset prevalence during the early phase of autoimmune and Trypanosoma cruzi-elicited myocarditis. Mem Inst Oswaldo Cruz. 2003;98:945-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Moretti E, Basso B, Cervetta L, Brigada A, Barbieri G. Patterns of cytokines and soluble cellular receptors in the sera of children with acute chagas’ disease. Clin Diagn Lab Immunol. 2002;9:1324-1327. [PubMed] [Cited in This Article: ] |
| 46. | Samudio M, Montenegro-James S, de Cabral M, Martinez J, Rojas de Arias A, Woroniecky O, James MA. Differential expression of systemic cytokine profiles in Chagas’ disease is associated with endemicity of Trypanosoma cruzi infections. Acta Trop. 1998;69:89-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Medrano NM, Luz MR, Cabello PH, Tapia GT, Van Leuven F, Araújo-Jorge TC. Acute Chagas’ disease: plasma levels of alpha-2-macroglobulin and C-reactive protein in children under 13 years in a high endemic area of Bolivia. J Trop Pediatr. 1996;42:68-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Gomes JA, Bahia-Oliveira LM, Rocha MO, Busek SC, Teixeira MM, Silva JS, Correa-Oliveira R. Type 1 chemokine receptor expression in Chagas’ disease correlates with morbidity in cardiac patients. Infect Immun. 2005;73:7960-7966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | Gomes JA, Bahia-Oliveira LM, Rocha MO, Martins-Filho OA, Gazzinelli G, Correa-Oliveira R. Evidence that development of severe cardiomyopathy in human Chagas’ disease is due to a Th1-specific immune response. Infect Immun. 2003;71:1185-1193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 222] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 50. | Araujo FF, Gomes JA, Rocha MO, Williams-Blangero S, Pinheiro VM, Morato MJ, Correa-Oliveira R. Potential role of CD4+CD25HIGH regulatory T cells in morbidity in Chagas disease. Front Biosci. 2007;12:2797-2806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | Reis DD, Jones EM, Tostes S, Lopes ER, Chapadeiro E, Gazzinelli G, Colley DG, McCurley TL. Expression of major histocompatibility complex antigens and adhesion molecules in hearts of patients with chronic Chagas’ disease. Am J Trop Med Hyg. 1993;49:192-200. [PubMed] [Cited in This Article: ] |
| 52. | Reis MM, Higuchi Mde L, Benvenuti LA, Aiello VD, Gutierrez PS, Bellotti G, Pileggi F. An in situ quantitative immunohistochemical study of cytokines and IL-2R+ in chronic human chagasic myocarditis: correlation with the presence of myocardial Trypanosoma cruzi antigens. Clin Immunol Immunopathol. 1997;83:165-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 124] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 53. | Ferreira RC, Ianni BM, Abel LC, Buck P, Mady C, Kalil J, Cunha-Neto E. Increased plasma levels of tumor necrosis factor-alpha in asymptomatic/”indeterminate” and Chagas disease cardiomyopathy patients. Mem Inst Oswaldo Cruz. 2003;98:407-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 54. | Talvani A, Rocha MO, Barcelos LS, Gomes YM, Ribeiro AL, Teixeira MM. Elevated concentrations of CCL2 and tumor necrosis factor-alpha in chagasic cardiomyopathy. Clin Infect Dis. 2004;38:943-950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 55. | Aliberti JC, Cardoso MA, Martins GA, Gazzinelli RT, Vieira LQ, Silva JS. Interleukin-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infect Immun. 1996;64:1961-1967. [PubMed] [Cited in This Article: ] |
| 56. | Torrico F, Heremans H, Rivera MT, Van Marck E, Billiau A, Carlier Y. Endogenous IFN-gamma is required for resistance to acute Trypanosoma cruzi infection in mice. J Immunol. 1991;146:3626-3632. [PubMed] [Cited in This Article: ] |
| 57. | Gazzinelli RT, Oswald IP, James SL, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol. 1992;148:1792-1796. [PubMed] [Cited in This Article: ] |
| 58. | Muñoz-Fernández MA, Fernández MA, Fresno M. Activation of human macrophages for the killing of intracellular Trypanosoma cruzi by TNF-alpha and IFN-gamma through a nitric oxide-dependent mechanism. Immunol Lett. 1992;33:35-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 144] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 59. | Fonseca SG, Reis MM, Coelho V, Nogueira LG, Monteiro SM, Mairena EC, Bacal F, Bocchi E, Guilherme L, Zheng XX. Locally produced survival cytokines IL-15 and IL-7 may be associated to the predominance of CD8+ T cells at heart lesions of human chronic Chagas disease cardiomyopathy. Scand J Immunol. 2007;66:362-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Graefe SE, Jacobs T, Gaworski I, Klauenberg U, Steeg C, Fleischer B. Interleukin-12 but not interleukin-18 is required for immunity to Trypanosoma cruzi in mice. Microbes Infect. 2003;5:833-839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Müller U, Köhler G, Mossmann H, Schaub GA, Alber G, Di Santo JP, Brombacher F, Hölscher C. IL-12-independent IFN-gamma production by T cells in experimental Chagas’ disease is mediated by IL-18. J Immunol. 2001;167:3346-3353. [PubMed] [Cited in This Article: ] |
| 62. | Silva JS, Twardzik DR, Reed SG. Regulation of Trypanosoma cruzi infections in vitro and in vivo by transforming growth factor beta (TGF-beta). J Exp Med. 1991;174:539-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 221] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 63. | dos Santos RR, Rossi MA, Laus JL, Silva JS, Savino W, Mengel J. Anti-CD4 abrogates rejection and reestablishes long-term tolerance to syngeneic newborn hearts grafted in mice chronically infected with Trypanosoma cruzi. J Exp Med. 1992;175:29-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 117] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 64. | Hölscher C, Mohrs M, Dai WJ, Köhler G, Ryffel B, Schaub GA, Mossmann H, Brombacher F. Tumor necrosis factor alpha-mediated toxic shock in Trypanosoma cruzi-infected interleukin 10-deficient mice. Infect Immun. 2000;68:4075-4083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 65. | da Matta Guedes PM, Gutierrez FR, Maia FL, Milanezi CM, Silva GK, Pavanelli WR, Silva JS. IL-17 produced during Trypanosoma cruzi infection plays a central role in regulating parasite-induced myocarditis. PLoS Negl Trop Dis. 2010;4:e604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 66. | Machado FS, Koyama NS, Carregaro V, Ferreira BR, Milanezi CM, Teixeira MM, Rossi MA, Silva JS. CCR5 plays a critical role in the development of myocarditis and host protection in mice infected with Trypanosoma cruzi. J Infect Dis. 2005;191:627-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 67. | Guedes PM, Veloso VM, Talvani A, Diniz LF, Caldas IS, Do-Valle-Matta MA, Santiago-Silva J, Chiari E, Galvão LM, Silva JS. Increased type 1 chemokine expression in experimental Chagas disease correlates with cardiac pathology in beagle dogs. Vet Immunol Immunopathol. 2010;138:106-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 68. | Garg N, Popov VL, Papaconstantinou J. Profiling gene transcription reveals a deficiency of mitochondrial oxidative phosphorylation in Trypanosoma cruzi-infected murine hearts: implications in chagasic myocarditis development. Biochim Biophys Acta. 2003;1638:106-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 69. | Vyatkina G, Bhatia V, Gerstner A, Papaconstantinou J, Garg N. Impaired mitochondrial respiratory chain and bioenergetics during chagasic cardiomyopathy development. Biochim Biophys Acta. 2004;1689:162-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 70. | Mukherjee S, Belbin TJ, Spray DC, Iacobas DA, Weiss LM, Kitsis RN, Wittner M, Jelicks LA, Scherer PE, Ding A. Microarray analysis of changes in gene expression in a murine model of chronic chagasic cardiomyopathy. Parasitol Res. 2003;91:187-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Riol-Blanco L, Sánchez-Sánchez N, Torres A, Tejedor A, Narumiya S, Corbí AL, Sánchez-Mateos P, Rodríguez-Fernández JL. The chemokine receptor CCR7 activates in dendritic cells two signaling modules that independently regulate chemotaxis and migratory speed. J Immunol. 2005;174:4070-4080. [PubMed] [Cited in This Article: ] |
| 72. | Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, Kaneko S. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci USA. 2006;103:14098-14103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 73. | Reddy VS, Harskamp RE, van Ginkel MW, Calhoon J, Baisden CE, Kim IS, Valente AJ, Chandrasekar B. Interleukin-18 stimulates fibronectin expression in primary human cardiac fibroblasts via PI3K-Akt-dependent NF-kappaB activation. J Cell Physiol. 2008;215:697-707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 74. | Kolattukudy PE, Quach T, Bergese S, Breckenridge S, Hensley J, Altschuld R, Gordillo G, Klenotic S, Orosz C, Parker-Thornburg J. Myocarditis induced by targeted expression of the MCP-1 gene in murine cardiac muscle. Am J Pathol. 1998;152:101-111. [PubMed] [Cited in This Article: ] |
| 75. | Kubota T, Bounoutas GS, Miyagishima M, Kadokami T, Sanders VJ, Bruton C, Robbins PD, McTiernan CF, Feldman AM. Soluble tumor necrosis factor receptor abrogates myocardial inflammation but not hypertrophy in cytokine-induced cardiomyopathy. Circulation. 2000;101:2518-2525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 76. | Johnston DL, Lewandowski ED. Fatty acid metabolism and contractile function in the reperfused myocardium. Multinuclear NMR studies of isolated rabbit hearts. Circ Res. 1991;68:714-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 77. | Carvajal K, Moreno-Sánchez R. Heart metabolic disturbances in cardiovascular diseases. Arch Med Res. 2003;34:89-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 78. | Wang D, McMillin JB, Bick R, Buja LM. Response of the neonatal rat cardiomyocyte in culture to energy depletion: effects of cytokines, nitric oxide, and heat shock proteins. Lab Invest. 1996;75:809-818. [PubMed] [Cited in This Article: ] |
| 79. | Kalovidouris AE, Plotkin Z, Graesser D. Interferon-gamma inhibits proliferation, differentiation, and creatine kinase activity of cultured human muscle cells. II. A possible role in myositis. J Rheumatol. 1993;20:1718-1723. [PubMed] [Cited in This Article: ] |
| 80. | Teixeira PC, Frade AP, Nogueira LG, Kalil J, Chevillard C, Cunha-Neto E. Pathogenesis of chagas disease cardiomyopathy. World J Clin Infect Dis. 2012;2:39-53. [DOI] [Cited in This Article: ] [Cited by in CrossRef: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (1)] |