Published online Jun 7, 2014. doi: 10.3748/wjg.v20.i21.6608
Revised: February 20, 2014
Accepted: March 12, 2014
Published online: June 7, 2014
AIM: To evaluate the value of positron emission tomography (PET)/computerized tomography (CT) in surveillance of colorectal cancer (CRC) patients with different carcinoembryonic antigen (CEA) concentrations.
METHODS: One hundred and six postoperative CRC patients who had suspected recurrence or metastasis and received fluorodeoxyglucose (FDG) PET/CT within one week were included in this study. The final diagnosis was confirmed by histological examination or clinical follow-up over at least six months.
RESULTS: The sensitivity, specificity, and accuracy of FDG PET/CT were 95.2%, 82.6%, and 92.5%, and 94.8%, 81.4% and 92.8%, respectively, in the case- and lesion-based analyses. The sensitivity and accuracy of FDG PET/CT significantly differed from CT in both analyses (χ2 = 8.186, P = 0.004; χ2 =6.201, P = 0.013; χ2 =13.445, P = 0.000; χ2 =11.194, P = 0.001). In the lesion-based analysis, the sensitivity, specificity, and accuracy of FDG PET/CT in the abnormal CEA group were 97.8%, 82.6%, and 95.6%, compared with 81.3%, 80%, and 80.6% for patients with normal CEA levels. In case-based analysis, the sensitivity, specificity, and accuracy of FDG PET/CT were 97.2%, 77.8%, and 95% in abnormal CEA group. Only in lesion-based analysis, the sensitivity and accuracy of FDG PET/CT in the abnormal CEA group were significantly superior to those in the normal CEA group (χ2 =6.432, P = 0.011; χ2 =7.837, P = 0.005). FDG PET/CT changed the management in 45.8% of patients with positive scans.
CONCLUSION: FDG PET/CT showed superior diagnostic value and is an advisable option in surveillance of postoperative CRC patients with a vague diagnosis.
Core tip: In this paper, fluorodeoxyglucose (FDG) positron emission tomography (PET)/computerized tomography (CT) showed an excellent diagnostic performance and its sensitivity and accuracy were significantly superior to those of CT. FDG PET/CT changed the management in some metastatic patients who might obtain the chance for a second remission. The study also showed that FDG PET/CT was effective similarly in the patients with normal and abnormal carcinoembryonic antigen (CEA) levels but had a tendency to increase with the CEA level. FDG PET/CT was an advisable option for surveillance of postoperative colorectal cancer (CRC) patients with a vague diagnosis and should be recommended in surveillance of post-operative CRC patients even with normal CEA.
- Citation: Zhang Y, Feng B, Zhang GL, Hu M, Fu Z, Zhao F, Zhang XL, Kong L, Yu JM. Value of 18F-FDG PET-CT in surveillance of postoperative colorectal cancer patients with various carcinoembryonic antigen concentrations. World J Gastroenterol 2014; 20(21): 6608-6614
- URL: https://www.wjgnet.com/1007-9327/full/v20/i21/6608.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i21.6608
Colorectal cancer (CRC) is one of the most common cancer entities worldwide[1]. Despite the fact that 70% of the patients have a chance of radical operation, 30%-50% of them will develop metastasis or local recurrence within two years after operation[2,3]. For CRC patients, both local recurrence and metastasis can be addressed by reoperation. Only 10%-30% of recurrent patients can be treated by salvage surgery[4,5], but curative-intent surgeries are associated with a 5-year survival rate of 30%-40% in selected patient populations with single organ metastasis[6]. Thus, the surveillance of postoperative colorectal cancer should enhance the proportion of resectable cases by early detection of the recurrence and metastasis in order to improve the survival of CRC patients.
The surveillance is usually performed by a regular physical examination, determination of the serum carcinoembryonic antigen (CEA) level, colonoscopy, and conventional imaging techniques such as ultrasound of the liver, contrast-enhanced computerized tomography (ceCT) and magnetic resonance imaging (MRI). It remains unverified what strategy can provide significant survival benefits in routine follow-up of CRC patients[7,8]. Serum CEA is generally used as a tumor marker and CEA elevation predicts a high risk of recurrence and poor survival for CRC[9]. Elevated CEA values can not provide any accurate information regarding the sites of recurrence and complementary imaging techniques should be provided for the diagnosis of CRC recurrence. Conventional imaging techniques primarily offer morphologic data based on anatomical information. It is difficult to identify recurrent disease from nonmalignant changes, such as scars, inflammation lesions and radiation necrosis by this morphological imaging tool. Fluorine-18 (18F) fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT is an integrated imaging modality of anatomic and functional imaging and can show metabolic changes before morphological ones. In the literature, 18F-FDG PET/CT was considered to be superior to the conventional imaging in the early detection of recurrence and metastases in CRC patients with elevated CEA[2,10]. Recently, it has been reported that PET/CT showed a high-positive predictive value for metastases in postoperative colorectal cancer patients with normal CEA levels[6,11]. However, due to some limitations in previous studies, such as small number of patients and unsatisfactory statistic analysis, the clinical value and efficacy of FDG PET/CT in surveillance are not yet fully established.
In this retrospective study, we aimed to evaluate the diagnostic performance of 18F-FDG PET/CT in surveillance of postoperative CRC patients as compared with CT and to investigate the role of FDG PET/CT in patients with different CEA concentrations.
A total of 106 postoperative CRC patients who underwent FDG PET/CT examinations at our institution from January 2008 to April 2012 were included in this study. The inclusion criteria were as follows: (1) histopathologic confirmation of primary CRC; (2) undergoing complete treatment including curative resection with or without chemoradiation therapy; (3) regular clinical examination every three or six months, including physical examinations, determination of serum CEA concentration, and chest and abdomen ceCT; (4) the patients suspected with recurrence or metastasis by routine examination received FDG PET/CT within one week; and (5) at least six months of clinical follow-up. Patient’s characteristics and other preoperative information are summarized in Table 1. This study was approved by the Institutional Review Board at our institution. Informed consent was waived due to the retrospective design of the study.
| Characteristic | n |
| Male/female | 71/35 |
| Age (yr, median) | 27-75 (56) |
| Primary site | |
| Colon | 42 |
| Rectum | 64 |
| Pathological type | |
| Adenocarcinoma | 94 |
| Adenosquamous carcinoma | 2 |
| Squamous cell carcinoma | 1 |
| Mucinous adenocarcinoma | 9 |
| Therapy | |
| Operation | 21 |
| Operation and chemotherapy | 13 |
| Operation and radiotherapy | 37 |
| Operation, chemotherapy and radiotherapy | 35 |
The 18F-FDG PET/CT was performed using an integrated PET/CT system (GE Discovery LS, GE Healthcare). All patients fasted for at least 6 h before the injection of 5 MBq/kg of 18F-FDG. Images were obtained approximately 1 h after an intravenous injection of FDG. The PET/CT system was used for 4-slice helical CT acquisition, followed by a full-ring dedicated PET scan of the same axial range. PET scans were performed in the whole-body mode from top to the middle thigh for 4 min per field of view, each covering 14.5 cm, at an axial sampling thickness of 4.25 mm/slice. PET images were reconstructed with CT-derived attenuation correction using ordered-subset expectation maximization software. The attenuation-corrected PET images, CT images, and fused PET/CT images were available for review in axial, coronal, sagittal planes, and a cine display of maximum intensity projections of the PET data, using the manufacturer’s review station (Xeleris; GE Healthcare).
The attenuation-corrected PET images, CT images, and fused PET/CT images displayed as coronal, sagittal, and transaxial slices were viewed on a Xeleris workstation. Two experienced nuclear medicine physicians, who were aware of the patient’s clinical history and recent radiographic data, interpreted the PET/CT images side-by-side using visual observation and semi-quantity analysis. It was considered positive when the maximum standard uptake value (SUVmax) of the region of interest (ROI) exceeded 2.5.
Serum CEA concentration was determined by electrochemiluminescence immunoassay with normal reference ranging from 0 to 3.4 ng/mL. It was categorized as abnormal when it exceeded 3.4 ng/mL. The patients were divided into four groups according to the CEA levels: group 1 (CEA ≤ 3.4 ng/mL), group 2 (CEA 3.4-10 ng/mL), group 3 (CEA 10-30 ng/mL), and group 4 (CEA > 30 ng/mL).
The final diagnosis of recurrence or metastasis was confirmed by gold standard (histopathological or cytological confirmation or at least six months of clinical follow-up). PET/CT findings and ceCT findings were classified as true positive (TP), false positive (FP), true negative (TN), and false negative (FN), as compared to those of the gold standard. The sensitivity, specificity, and accuracy of 18F-FDG PET/CT and ceCT were calculated using standard statistical formula in the case-based and lesion-based analyses.
The SPSS version 17.0 (SPSS Inc, Chicago, IL, United States) was used for statistical analyses. The Chi-square test was used to compare the differences between the two imaging modalities.
In the group, 51 patients were confirmed by histocytology while 55 patients by follow-up. In the case-based analysis, 83 patients had positive findings in PET/CT and 79 patients were finally diagnosed as TP. Nineteen patients were identified as TN, including four patients with benign diseases (one thyroid adenoma and three enteric polyps). Among the four FP patients, two were suspected to have recurrence at the anastomotic site and finally confirmed to have inflammatory changes by colonoscopy. In two FN patients, tiny peritoneum and lung metastases were revealed with a diameter less than 0.5 cm, which were not visualized by PET/CT but confirmed by ceCT imaging several months later when the diameter was larger. With little FDG uptake, two mucinous adenocarcinoma patients showed negative PET/CT scans and were determined as FN by histological confirmation. In all patients, 67 TP patients and 17 TN patients were diagnosed by ceCT and there were intersections of PET/CT and ceCT (Table 2).
| CT (n = 106) | PET/CT (n = 106) | |||
| TP (n = 79) | FP (n = 4) | TN (n = 19) | FN (n = 4) | |
| TP (n = 67) | 67 | |||
| FP (n = 6) | 4 | 2 | ||
| TN (n = 17) | 17 | |||
| FN (n = 16) | 12 | 4 | ||
In the lesion-based analysis, out of the 152 positive lesions determined by 18F-FDG PET/CT, 146 were confirmed as TP lesions and six as FN lesions by gold standard. Thirty five lesions were confirmed as TN scans and eight lesions were identified as FP scans. The locations of the foci included anastomotic site, intraperitoneal and thoracic lymph nodes, pelvic, bone, liver, and lungs. Three FP lesions showed nonspecific FDG uptake, which were confirmed as inflammatory changes in the wall of the bowel or around operation site, and five lesions had hypermetabolic lesions in the liver, lung, pelvic, and bone. Three FN lesions were mucinous adenocarcinoma and the others were mediastinal lymph nodes and small nodules of the lung and peritoneum. The sensitivity and accuracy of FDG PET/CT significantly differed from those of ceCT in both case- and lesion-based analyses (P < 0.05, Table 3). As for the specificity, the PET/CT results did not show a significant difference from those of ceCT in both analyses.
| PET/CT and CT in all patients | PET/CT in patients with different CEA levels | |||
| PET/CT | CT | PET/CT (CEA ≤ 3.4 ng/mL) | PET/CT (CEA > 3.4 ng/mL) | |
| Case-based analysis | ||||
| Sensitivity (%) | 95.2 (79/83)1 | 80.7 (67/83)1 | 83.3 (10/12) | 97.2 (69/71) |
| Specificity (%) | 82.6 (19/23) | 73.9 (17/23) | 85.7 (12/14) | 77.8 (7/9) |
| Accuracy (%) | 92.5 (98/106)2 | 79.3 (84/106)2 | 84.6 (22/26) | 95 (76/80) |
| Lesion-based analysis | ||||
| Sensitivity (%) | 96.1 (146/152)3 | 83.1 (118/142)3 | 81.3 (13/16)5 | 97.8 (133/136)5 |
| Specificity (%) | 81.4 (35/43) | 73.5 (25/34) | 80 (16/20) | 82.6 (19/23) |
| Accuracy (%) | 92.8 (181/195)4 | 81.3 (143/176)4 | 80.6 (29/36)6 | 95.6 (152/159)6 |
We detected serum CEA concentration in all of the patients. In case-based analysis, the sensitivity, specificity, and accuracy of PET/CT were 97.2%, 77.8%, and 95%, respectively, in the group with abnormal CEA levels and did not significantly differ from those in the group with normal CEA levels. In lesion-based analysis, the sensitivity and accuracy of PET/CT in the group with abnormal CEA levels were significantly superior to those in the group with normal CEA levels (P < 0.05, Table 3). The diagnostic performance of FDG PET/CT and ceCT was calculated in each group with various CEA levels. The sensitivity, specificity, and accuracy of both imaging techniques were increased with the CEA level, but there was no significant statistical difference among the groups of patients with different CEA levels due to the small quantity of sample in each group (Table 4).
| CEA level (ng/mL) | CT (%) | PET/CT (%) | ||||
| Sensitivity | Specificity | Accuracy | Sensitivity | Specificity | Accuracy | |
| ≤ 3.4 (n = 26) | 57.1 (8/14) | 75 (9/12) | 65.4 (17/26) | 83.3 (10/12) | 85.7 (12/14) | 84.6 (22/26) |
| 3.4-10 (n = 29) | 78.3 (18/23) | 66.7 (4/6) | 75.9 (22/29) | 96 (24/25) | 75 (3/4) | 93.1 (27/29) |
| 10-30 (n = 34) | 86.7 (26/30) | 75 (3/4) | 85.3 (29/34) | 96.7 (29/30) | 75 (3/4) | 94.1 (32/34) |
| > 30 (n = 17) | 93.8 (15/16) | 100 (1/1) | 94.1 (16/17) | 100 (16/16) | 100 (1/1) | 100 (17/17) |
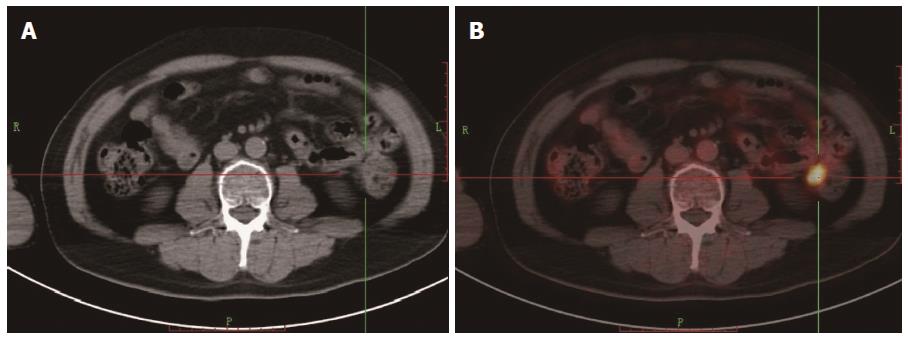
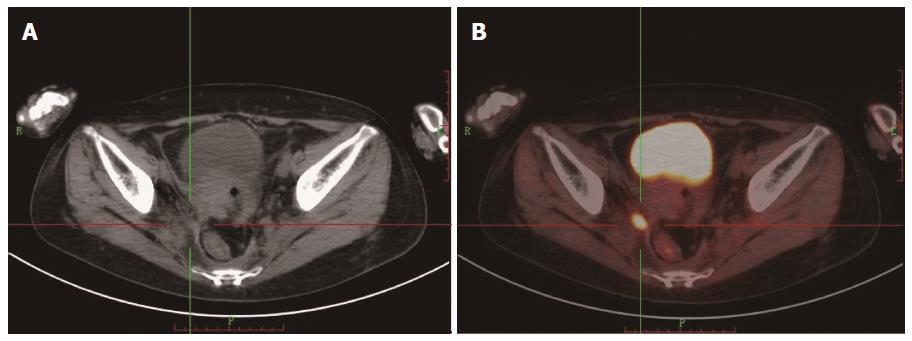
Among all the patients, 69 showed positive scans in both FDG PET/CT and ceCT, but 18 showed extra lesions in FDG PET/CT. In 12 patients, FDG PET/CT showed positive scans, while CT was negative (Figures 1 and 2). Of the patients with recurrence and metastasis, 57 received radiotherapy or/and chemotherapy. Ten patients received secondary surgery for a single metastatic lesion and eight patients had canceled operative scheme due to the observation of extra lesions by FDG PET/CT. Of the total 83 patients with positive scans, FDG PET/CT changed the management in 38 (45.8%) patients (Table 5).
| Surgery | Radiotherapy | Chemotherapy | Chemoradiotherapy | |
| Added cases (n = 23) | 6 | 3 | 9 | 5 |
| Reduced cases (n = 15) | 8 | 2 | 3 | 2 |
| Total (n = 38) | 14 | 5 | 12 | 7 |
In surveillance of postoperative CRC, CT is the preferred imaging technique for local recurrence detection[6] and MRI is regarded as the most sensitive conventional imaging tool for liver metastases[12,13]. However, the early diagnosis of recurrence is still difficult through conventional surveillance strategies. It has been reported that PET/CT showed better sensitivity and specificity (87%-100% and 90%-98%, respectively) for detection of hepatic and extra-hepatic metastasis than CT[14-16]. In some studies, the sensitivity of PET/CT for hepatic lesions was 91%-100%, which was similar to that of MRI[17,18]. In our study, 18F-FDG PET/CT showed an excellent diagnostic performance in the detection of CRC recurrence and metastasis and its sensitivity and accuracy were significantly superior to those of ceCT. We found that many positive lesions in FDG PET/CT, which were once classified as negative lesions by CT (Table 5), were finally confirmed as true positives by gold standard. This showed that it would be possible that some CRC patients with recurrences and metastasis might obtain the chance for a second remission and improve the prognosis through PET/CT findings.
CEA is used as the early indicator for the recurrent disease and is elevated in approximately 60%-70% of patients with recurrence[10,19]. It has been observed that some potentially curable recurrent tumors were detected by routine imaging techniques, while CEA levels were still normal. Therefore, CEA measurements had only a marginal effect on survival[11]. Moreover, for postoperative CRC patients who have a suspicion of recurrence based on the rise in the CEA level, there is controversy on the most accurate imaging technique. Ozkan et al[2] reported that the sensitivity and specificity of 18F-FDG PET/CT in the detection of disease recurrence in postoperative CRC patients were 97% and 61%, while they were 51% and 60% for CT, respectively. Mittal et al[20] reported that PET/CT showed recurrences in 71% of CRC patients and the positive rate increased with the CEA level. Our data also showed coincidentally that PET/CT was superior to CT in terms of sensitivity, specificity, and accuracy in all groups of patients and had a tendency to increase with the CEA level (Table 4). Lee et al[21] evaluated a group of CRC patients with normal CEA levels and reported that the sensitivity, specificity, and accuracy for FDG PET/CT were 95%, 76.6%, and 88.8%, respectively. In our study, the sensitivity, specificity, and accuracy of PET/CT in patients with normal CEA was similar to the data. In addition, the sensitivity and accuracy of FDG PET/CT in patients with abnormal CEA levels significantly differed from those with normal CEA levels in the lesion-based analysis. But in the case-based analysis, the FDG PET/CT diagnostic performance did not show a significant difference between these two groups. It may be related to the small quantity of patients with normal CEA levels, which could influence the statistical analysis. However, the data still suggested that FDG PET/CT was effective similarly in patients with normal and abnormal CEA levels. For patients with a vague diagnosis of recurrence or metastasis based on a routine examination, FDG PET/CT might provide much benefit to patients by increasing the diagnostic accuracy. Based upon these results, FDG PET/CT is an advisable option for CRC patients with an indefinable diagnosis and should be recommended in surveillance of post-operative CRC patients even with normal CEA.
In this study, the specificity of FDG PET/CT did not significantly differ from that of ceCT in both case- and lesion-based analyses. FDG PET/CT scans showed false-positive results which mainly included the inflammatory lesion and single hypermetabolic lesion in the organs. Our data demonstrated that inflammatory processes can result in hypermetabolism and consequently false-positive results, for example, the inflammatory processes of colitis in the anastomotic site. In addition, for a single hypermetabolic lesion of the bone, metastasis should be differentiated from the injuries and restorations correlated to other imaging systems. For lung nodules, metastasis should be differentiated from inflammatory lesions and tuberculosis.
It has been reported that mucinous carcinoma usually showed little uptake of the FDG and the sensitivity of FDG-PET imaging for detection of mucinous carcinoma was significantly lower than that for nonmucinous carcinomas[3]. In our results, two quarters of FN cases and three sixths of FN lesions were mucinous adenocarcinoma and it demonstrated that mucinous carcinoma was the main factor responsible for FN scans. Therefore, it was suggested that for mucinous adenocarcinoma patients with negative FDG-PET imaging results, other imaging modalities should be recommended for further diagnosis. Otherwise, nonvisualization of FDG PET/CT detection should be attributed to the little FDG uptake for lymph nodes and peritoneal micrometastases, consistent with the literature report. Moreover, our FDG PET/CT scanning data were acquired in a 2D mode with 4.25 mm spatial resolution and a 256 x 256 matrix. Given better spatial and temporal resolution (3D mode with 2 mm spatial resolution, 400 x 400 matrix and continuous table movement), more tiny lymph nodes and metastases can be revealed precisely. With the decrease of false diagnosis, the further accuracy elevation can be expected.
In terms of the superiority of FDG PET/CT over CT in detection of recurrence and metastasis, FDG PET/CT might provide chances to select suitable patients for surgical resection or other local treatments (radiotherapy, embolization, and radio-frequency ablation). Meanwhile, some unnecessary operations might be avoided. Many studies demonstrated some disease management change as a result of PET/CT usage in 30%-56% of patients with a suspected or confirmed recurrence of CRC[22-24]. It is possible to benefit from this strategy in terms of patient survival with early detection and treatment of tumor recurrence by FDG PET/CT[6,25]. In our studies, 45.8% of FDG positive patients had a changed disease management and received suitable treatments. Second operation was performed only in 12% of retreatment candidate patients, because most patients of the groups had already developed multi-organ metastases when they received the FDG PET/CT scan. It suggested that the early detection due to the use of PET/CT in surveillance of CRC could correct the disease management strategy and improve the treatment efficacy by reoperation for potential curative patients.
Our study had two potential limitations. Due to the retrospective nature of the study, we were unable to obtain unified clinical date for the patients and there was an inter-observer variation for imaging interpretation that might have had some influence on the sequences. The second was that histopathological confirmation was only performed in some of the patients in this study.
In conclusion, FDG PET/CT showed a superior diagnostic performance in surveillance of postoperative CRC patients. For patients with suspicious recurrence or metastasis based on a routine examination, our data suggest that PET/CT is an excellent option to replace CT in the follow-up of CRC patients even when CEA is normal.
The authors thank Dr. Hui-Qing Li and Yuan-Yuan Liu for their assistance with this paper.
Recurrent or metastatic colorectal cancer (CRC) patients will have a chance of remission by early detection in postoperative surveillance and improve the survival. It remains unverified which strategy can provide significant survival benefits in routine surveillance of CRC patients. In the literature, fluorodeoxyglucose (FDG) positron emission tomography (PET)/computerized tomography (CT) was considered to be superior to conventional imaging in early detection of recurrence and metastasis in CRC patients. However, due to insufficient evidence, the clinical value of FDG PET/CT in surveillance is not definite.
Recently, as an integrated imaging modality of anatomic and functional imaging diagnosis, FDG PET/CT showed enormous potential in area of diagnosis, staging and monitoring and response evaluation of CRC. In the surveillance of postoperative CRC patients, the hotspots are whether PET/CT is more effective than conventional imaging such as CT and what kind of patients should be suitable candidates for PET/CT.
Previous studies usually compared the diagnostic performance of imaging modes in all patients regardless the carcinoembryonic antigen (CEA) level. In the current literature, the diagnostic performances of FDG PET/CT were evaluated only in patients with normal or abnormal CEA levels. In this retrospective study, the authors evaluated the diagnostic performance of Fluorine-18 (18F)-FDG PET/CT in surveillance of postoperative CRC patients as compared with CT and to investigate the role of FDG PET/CT in patients with different CEA levels. The data showed that PET/CT was superior to CT in terms of sensitivity, specificity, and accuracy in all groups of patients. The data also suggested that FDG PET/CT was effective in patients with normal or abnormal CEA levels.
The study suggested that PET/CT was an excellent option to replace CT in surveillance of CRC patients who were suspected to have recurrence or metastasis by routine examinations even when CEA is normal.
18F-FDG PET/CT is an integrated imaging modality of anatomic and functional imaging and can show metabolic changes before morphological ones. It depicts the spatial distribution of metabolic or biochemical activity in the body and has excellent diagnostic performance in various tumors.
This is a good retrospective study in which the authors compared the diagnostic performance of 18F-FDG PET/CT with CT in surveillance of postoperative CRC patients and assessed the value of FDG PET/CT in patients with normal or abnormal CEA levels.
P- Reviewers: Grassetto G, Luboldt W, Martinez-Zorzano VS S- Editor: Zhai HH L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9215] [Cited by in F6Publishing: 9719] [Article Influence: 883.5] [Reference Citation Analysis (3)] |
| 2. | Ozkan E, Soydal C, Araz M, Kir KM, Ibis E. The role of 18F-FDG PET/CT in detecting colorectal cancer recurrence in patients with elevated CEA levels. Nucl Med Commun. 2012;33:395-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Borasio P, Gisabella M, Billé A, Righi L, Longo M, Tampellini M, Ardissone F. Role of surgical resection in colorectal lung metastases: analysis of 137 patients. Int J Colorectal Dis. 2011;26:183-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Hirai I, Kimura W, Fuse A, Isobe H, Hachiya O, Moriya T, Suto K, Mizutani M. Surgical management for metastatic liver tumors. Hepatogastroenterology. 2006;53:757-763. [PubMed] [Cited in This Article: ] |
| 5. | Schaefer O, Langer M. Detection of recurrent rectal cancer with CT, MRI and PET/CT. Eur Radiol. 2007;17:2044-2054. [PubMed] [Cited in This Article: ] |
| 6. | Maas M, Rutten IJ, Nelemans PJ, Lambregts DM, Cappendijk VC, Beets GL, Beets-Tan RG. What is the most accurate whole-body imaging modality for assessment of local and distant recurrent disease in colorectal cancer? A meta-analysis: imaging for recurrent colorectal cancer. Eur J Nucl Med Mol Imaging. 2011;38:1560-1571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Koo SL, Wen JH, Hillmer A, Cheah PY, Tan P, Tan IB. Current and emerging surveillance strategies to expand the window of opportunity for curative treatment after surgery in colorectal cancer. Expert Rev Anticancer Ther. 2013;13:439-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Li Destri G, Di Cataldo A, Puleo S. Colorectal cancer follow-up: useful or useless? Surg Oncol. 2006;15:1-12. [PubMed] [Cited in This Article: ] |
| 9. | Szynglarewicz B, Matkowski R, Forgacz J, Pudelko M, Smorag Z, Dryl J, Kornafel J. Clinical factors in prediction of prognosis after anterior resection with total mesorectal excision for carcinoma of the rectum. Oncol Rep. 2007;17:471-475. [PubMed] [Cited in This Article: ] |
| 10. | Fiocchi F, Iotti V, Ligabue G, Malavasi N, Luppi G, Bagni B, Torricelli P. Role of carcinoembryonic antigen, magnetic resonance imaging, and positron emission tomography-computed tomography in the evaluation of patients with suspected local recurrence of colorectal cancer. Clin Imaging. 2011;35:266-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Tan E, Gouvas N, Nicholls RJ, Ziprin P, Xynos E, Tekkis PP. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surg Oncol. 2009;18:15-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 12. | Floriani I, Torri V, Rulli E, Garavaglia D, Compagnoni A, Salvolini L, Giovagnoni A. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: a systematic review and meta-analysis. J Magn Reson Imaging. 2010;31:19-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 13. | Morton KA, Clark PB. Diagnostic imaging nuclear medicine. 8th ed. Utah: Amirsys 2007; . [Cited in This Article: ] |
| 14. | Shamim SA, Kumar R, Halanaik D, Shandal V, Reddy RM, Bal CS, Malhotra A. Role of FDG-PET/CT in detection of recurrent disease in colorectal cancer. Nucl Med Commun. 2010;31:590-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Kyoto Y, Momose M, Kondo C, Itabashi M, Kameoka S, Kusakabe K. Ability of 18F-FDG PET/CT to diagnose recurrent colorectal cancer in patients with elevated CEA concentrations. Ann Nucl Med. 2010;24:395-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Chiewvit S, Jiranantanakorn T, Apisarnthanarak P, Kanchaanapiboon P, Hannanthawiwat C, Ubolnuch K, Phongsawat N, Chiewvit P. Detection of recurrent colorectal cancer by 18F-FDG PET/CT comparison with contrast enhanced CT scan. J Med Assoc Thai. 2013;96:703-708. [PubMed] [Cited in This Article: ] |
| 17. | Patel S, McCall M, Ohinmaa A, Bigam D, Dryden DM. Positron emission tomography/computed tomographic scans compared to computed tomographic scans for detecting colorectal liver metastases: a systematic review. Ann Surg. 2011;253:666-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology. 2010;257:674-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 420] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 19. | Hara M, Kanemitsu Y, Hirai T, Komori K, Kato T. Negative serum carcinoembryonic antigen has insufficient accuracy for excluding recurrence from patients with Dukes C colorectal cancer: analysis with likelihood ratio and posttest probability in a follow-up study. Dis Colon Rectum. 2008;51:1675-1680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Mittal BR, Senthil R, Kashyap R, Bhattacharya A, Singh B, Kapoor R, Gupta R. 18F-FDG PET-CT in evaluation of postoperative colorectal cancer patients with rising CEA level. Nucl Med Commun. 2011;32:789-793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Lee JH, Park SG, Jee KN, Park DG, Namgung H, Song IH. Performance of FDG PET/CT in postoperative colorectal cancer patients with a suspected recurrence and a normal CEA level. Nucl Med Commun. 2010;31:576-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | El-Maghraby T. PET and PET/CT in the clinical management of colorectal cancer. Gulf J Oncolog. 2009;8-16. [PubMed] [Cited in This Article: ] |
| 23. | Kitajima K, Murakami K, Yamasaki E, Domeki Y, Tsubaki M, Sunagawa M, Kaji Y, Suganuma N, Sugimura K. Performance of integrated FDG PET/contrast-enhanced CT in the diagnosis of recurrent colorectal cancer: Comparison with integrated FDG PET/non-contrast-enhanced CT and enhanced CT. Eur J Nucl Med Mol Imaging. 2009;36:1388-1396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Faneyte IF, Dresen RC, Edelbroek MA, Nieuwenhuijzen GA, Rutten HJ. Pre-operative staging with positron emission tomography in patients with pelvic recurrence of rectal cancer. Dig Surg. 2008;25:202-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |