Abstract
Dosages of anticancer drugs are usually calculated on the basis of a uniform standard, the body surface area (BSA). Although many physiological functions are proportionate to BSA, overall drug clearance is only partially related to this parameter. Consequently, following administration of equivalent drug dosages based on BSA, a wide variability in plasma drug concentrations can be found between patients, as a result of which some patients experience little toxicity while others may show severe toxic symptoms. A clear pharmacokinetic/pharmacodynamic correlation has been demonstrated for some anticancer drugs, and this relationship provides a background against which rational dose optimisation can be implemented for individual patients. The 3 strategies that can be employed for optimising dosage regimens, none based on BSA, are described and criticised.
A priori adaptive dosage determination is based on the relative contribution of identifiable characteristics of patient, drug therapy and disease state that influence plasma drug concentrations; the dosage regimen is based on each patient’s profile with regard to these characteristics. Although this approach is most successful with drugs whose clearance is closely tied to renal function, patient characteristics such age, obesity, serum albumin or hepatic function may be useful. The anticancer drug most closely identified with this approach is carboplatin, although dosage reduction strategies for etoposide, taxanes, anthracyclines, topotecan, oxazaphosphorines, vinca alkaloids or melphalan are advocated for patients with renal or hepatic dysfunction. The importance of pharmacogenetics for fluorouracil and mercaptopurine is also briefly discussed.
The second approach consists of adaptive dosage adjustments during repetitive or continuous administration of a drug. It has been used for several years to administer methotrexate therapy and, more recently, it has been developed more fully and applied to continuous infusion of fluorouracil or etoposide. It was based, after determination of a target plasma concentration or area under the plasma drug concentration-time curve (AUC), on modification of the drug dosage during the cycle of chemotherapy or for the next cycle.
Finally, the third approach of adaptive dosage adjustment with feedback control, based on population pharmacokinetics, with limited sampling strategy, may allow a feedback revision of the dosage following measurement of plasma drug concentration and comparison with the population previously studied. This approach is a theoretical strategy which has not, until now, been used prospectively in clinical oncology.
For drugs such as anticancer agents with a very narrow therapeutic index, every effort should be made to minimise interpatient variability in drug exposure in order to maximise the benefit while keeping the risk of serious adverse effects at an acceptable level. This is particularly important when treatment is being given with curative intent.
Similar content being viewed by others
References
Egorin MJ. Therapeutic drug monitoring and dose optimisation in oncology. In: Workman P, editor. New approaches in cancer pharmacology: drug design and development. Berlin,: Springer-Verlag, 1992: 75–87
Newell DR. Can pharmacokinetic and pharmacodynamic studies improve cancer chemotherapy? [discussion no. 15]. Ann Oncol 1994; 5 Suppl. 4: 9–14
Moore MJ, Erlichman C. Therapeutic drug monitoring in oncology: problems and potential in antineoplastic therapy. Clin Pharmacokinet 1987; 13: 205–7
Egorin MJ, Reyno LM, Canetta RM, et al. Modeling toxicity and response in carboplatin-based combination chemotherapy. Semin Oncol 1994; 5 Suppl. 12: 7–19
Ratain MJ, Schilsky RL, Conley BA, et al. Pharmacodynamics in cancer therapy. J Clin Oncol 1990; 8: 1739–53
Evans WE, Relling MV. Clinical pharmacokinetics-pharmaco-dynamics of anticancer drugs. Clin Pharmacokinet 1989; 16: 327–6
Kobayashi K, Ratain MJ. New perspectives on the toxicity of etoposide. Semin Oncol 1992; 19: 78–83
Kobayashi K, Ratain MJ. Individualizing dosing of cancer chemotherapy. Semin Oncol 1993; 20: 30–42
Masson E, Zamboni WC. Pharmacokinetic optimisation of cancer chemotherapy: effect on outcomes. Clin Pharmacokinet 1997; 32: 324–43
Horwich A, Dearnley DP, Nicholls J. Effectiveness of carboplatin, etoposide and bleomycin combination chemotherapy in good-prognosis metastatic germ cell tumours. J Clin Oncol 1991; 9: 62–9
Jodrell D, Egorin MJ, Canetta RM. Relationships between carboplatin exposure and tumor response and toxicity in patients with ovarian cancer. J Clin Oncol 1992; 10: 520–8
Ayash LJ, Wright JE, Tretyakov O, et al. Cyclophosphamide pharmacokinetics: correlation with cardiac toxicity and tumour response. J Clin Oncol 1992; 10(6): 995–1000
Kantarjian HM, Estey EH, Plunkett W, et al. Phase I/II clinical and pharmacologic studies of high dose cytosine arabinoside in refractory leukemia. Am J Med 1986; 81: 387–94
Desoize B, Marechal F, Cattan A. Clinical pharmacokinetics of etoposide during 120 hours continuous infusion in solid tumours. Br J Cancer 1990; 62: 840–1
Milano G, Etienne MC, Renee N, et al. Relationship between fluorouracil systemic exposure and tumor response and patient survival. J Clin Oncol 1994; 12: 1291–5
Lennard L, Lilleyman JS. Variable mercaptopurine metabolism and treatment outcome in childhood lymphoblastic leukemia. J Clin Oncol 1989; 7: 1816–23
Lilleyman JS, Lennard L. Mercaptopurine metabolism and treatment outcome in childood lymphoblastic leukemia. Lancet 1994; 343(8907): 1188–90
Evans WE, Crom WR, Abromowitch M, et al. Clinical pharmacodynamics of high-dose methotrexate in acute lymphocytic leukemia: identification of a relation between concentration and effect. N Engl J Med 1988; 314: 471–77
Masson E, Relling MV, Synold TW, et al. Accumulation of methtrexate polyglutamates in lymphoblasts is a determinant of antileukemic effects in vivo. J Clin Invest 1996; 97: 73–80
Graf N, Winkler K, Betlemovic M, et al. Methotrexate pharmacokinetics and prognosis in osteosarcoma. J Clin Oncol 1994; 12: 1443–51
Rodman JH, Abromowitch M, Sinkule JA, et al. Clinical pharmacodynamics of continuous infusion teniposide: systemic exposure as a determinant of response in a phase I trial. J Clin Oncol 1987; 5: 1007–4
Crom WR, Glynn-Barnhart AM, Rodman JH, et al. Pharmacokinetics of anticancer drugs in children. Clin Pharmacokinet 1987; 12: 168–213
Evans WE. Clinical pharmacodynamics of anticancer drugs: a basis for extending the concept of dose-intensity. Blut 1988; 56: 241–8
Evans WE, Petros WP, Relling MV, et al. Clinical pharmacology of cancer chemotherapy in children. Pediatr Clin North Am 1989; 36: 1199–230
Petros WP, Evans WE. Pharmacokinetics and pharmacodynamics of anticancer agents: contributions to the therapy of childhood cancer. Pharmacotherapy 1990; 10: 313–25
Evans WE, Rodman JH, Relling MV, et al. Concept of maximum tolerated systemic exposure and its application to phase I-II studies of anticancer drugs. Med Pediatr Oncol 1991; 19: 153–9
McLeod HI, Evans WE. Pediatric pharmacokinetics and therapeutic drug monitoring. Pediatr Rev 1992; 13: 413–21
Evans WE. Alternative approaches for phase I studies of anti-cancer drugs: a role for therapeutic drug monitoring. Ther Drug Monit 1993; 15: 492–7
Crom WR. Pharmacokinetics in the child. Environ Health Persp 1994; 11: 111–7
Evans WE, Relling MV, Rodman JH, et al. Conventional compared with individualized chemotherapy for childhood acute lymphoblastic leukemia. N Engl J Med 1998; 338: 499–505
Egorin MJ, Van Echo DA, Tipping SJ, et al. Pharmacokinetics and dosage reduction of cis-diammine(l,l-cyclobutanedi-carboxylato) platinum in patients with impaired renal function. Cancer Res 1984; 44: 5432–8
Ratain MJ, Vogelzang NJ. Phase I and pharmacological study of vinblastine by prolonged continuous infusion. Cancer Res 1986; 46: 4827–30
Belani CP, Egorin MJ, Abrams JS, et al. A novel pharmaco-dynamically based approach to dose optimization of carbo-platin when used in combination with etoposide. J Clin Oncol 1989; 7: 1896–902
Newell DR, Eeles RA, Gumbrell LA, et al. Carboplatin and etoposide pharmacokinetics in patients with testicular teratoma. Cancer Chemother Pharmacol 1989; 23: 367–72
Tranchand B, Ploin YD, Minuit MP, et al. High-dose melphalan dosage adjustment: possibility of using a test-dose. Cancer Chemother Pharmacol 1989; 23: 95–100
Ratain MJ, Schilsky RL, Choi KE, et al. Adaptive control of etoposide administration: impact of interpatient pharmaco-dynamic variability. Clin Pharmacol Ther 1989; 45: 226–33
Ratain MJ. Dose reduction of etoposide in jaundiced patients. J Clin Oncol 1990; 8: 2088–9
Sorensen BT, Stromgren A, Jakobsen P, et al. Dose-toxicity relationship of carboplatin in combination with cyclophos-phamide in ovarian cancer patients. Cancer Chemother Pharmacol 1991; 28: 397–401
O’Dwyer P, LaCreta FP, Engstrom PF, et al. Phase I/pharmacokinetic reevaluation of ThioTEPA. Cancer Res 1991; 51: 3171–6
Jakobsen P, Bastolt L, Dalmark M, et al. Feasibility of myelotoxicity prediction through single blood sample measurement. Cancer Chemother Pharmacol 1991; 28: 465–8
Stewart CF, Arbuck SG, Fleming RA, et al. Relation of systemic exposure to unbound etoposide and hematologic toxicity. Clin Pharmacol Ther 1991; 50: 385–93
Evans WE, Rodman JH, Relling MV, et al. Differences in teniposide disposition and pharmacodynamics in patients with newly diagnosed and relapsed acute lumphocytic leukemia. J Pharmacol Exp Ther 1992; 260: 71–7
Ploin DY, Tranchand B, Guastalla JP, et al. Pharmacokinetically guided dosing for intravenous melphalan: a pilot study in patients with advanced ovarian adenocarcinoma. Eur J Cancer 1992; 28A: 1311–5
Miller AA, Tolley EA, Niell HB, et al. Pharmacodynamics of three daily infusions of etoposide in patients with extensivestage small-cell lung cancer. Cancer Chemother Pharmacol 1992; 31: 161–6
Miller AA, Tolley EA, Niell HB, et al. Pharmacodynamics of prolonged oral etoposide in patients with advanced non-small-cell lung cancer. J Clin Oncol 1993; 11: 1179–88
Pfluger KH, Hahn M, Holz JB, et al. Pharmacokinetics of etoposide: correlation of pharmacokinetic parameters with clinical conditions. Cancer Chemother Pharmacol 1993; 31: 350–6
Piscitelli SC, Rodvold KA, Rushing DA, et al. Pharmacokinetics and pharmacodynamics of doxorubicin in patients with small cell lung cancer. Clin Pharmacol Ther 1993; 53: 555–61
Rowinsky EK. Clinical pharmacology of taxol. J Natl Cancer Inst 1993; 15: 25
Minami H, Shimokata K, Saka H, et al. Phase I clinical and pharmacokinetic study of a 14-day infusion of etoposide in patients with lung cancer. J Clin Oncol 1993; 11: 1602–8
Fety R, Rolland F, Barberi-Heyob M, et al. Clinical impact of pharmacokinetically guided adaptation of 5-fluorouracil: results from a multicentre, randomized trial in patients with locally advanced head and neck carcinomas. Clin Cancer Res 1998; 4: 2039–45
Chatelut E, Chevreau C, Brunner V, et al. A pharmacologically guided phase I study of carboplatin in combination with methotrexate and vinblastine in advanced urothelial cancer. Cancer Chemother Pharmacol 1995; 35: 391–6
Kearns CM, Gianni L, Egorin MJ. Paclitaxel pharmacokinetics and pharmacodynamics. Semin Oncol 1995; 22: 16–23
Huizing MT, Vermorken JB, Rosing H, et al. Pharmacokinetics of paclitaxel and three major metabolites in patients with advanced breast carcinoma refractory to anthracycline therapy treated with a 3-hour paclitaxel infusion: a European Cancer Centre (ECC) trial. Ann Oncol 1995; 6: 699–704
van Wamerdam LJC, Verweij J, Schellens JH, et al. Pharmacokinetics and pharmacodynamics of topotecan administered daily for 5 days every three weeks. Cancer Chemother Pharmacol 1995; 35: 237–45
Millward MJ, Newell DR, Yuen K, et al. Pharmacokinetics and pharmacodynamics of prolonged oral etoposide in women with metastatic breast cancer. Cancer Chemother Pharmacol 1995; 37: 161–7
Chabot GG, Abigerges D, Catimel G, et al. Population pharmacokinetics and pharmacodynamics of irinotecan (CPT-11) and active metabolite SN-38 during phase I trials. Ann Oncol 1995; 6: 141–51
Canal P, Gay C, Dezeuze A, et al. Pharmacokinetics and pharmacodynamics of irinotecan-hydrochloride (CPT-11) during a phase II clinical trial in colorectal cancer. J Clin Oncol 1996; 14: 2688–95
Bruno R, Vivier N, Vergniol JC, et al. A population pharmacokinetic model for docetaxel (taxotere): model building and validation. J Pharmacokinet Biopharm 1996; 24: 153–72
Bruno R, Hill D, Riva A, et al. Population pharmacokinetics/pharmacodynamics of docetaxel in phase JJ studies in patients with cancer. J Clin Oncol 1998; 16: 187–96
Minami H, Ratain MJ, Ando Y, et al. Pharmacodynamic modeling of prolonged administration of etoposide. Cancer Chemother Pharmacol 1996; 39: 61–6
Joel SP, Ellis P, O’Byrne K, et al. Therapeutic monitoring of continuous infusion etoposide in small-cell lung cancer. J Clin Oncol 1996; 14: 1903–12
Joel S. The clinical pharmacology of etoposide: an update. Cancer Treat Rev 1996; 22: 179–221
Huizing MT, Giaccone G, van Warmerdam LJ, et al. Pharmacokinetics of paclitaxel and carboplatin in a dose-escalating and dose-sequencing study in patients with non-small-cell lung cancer: the European Cancer Centre. J Clin Oncol 1997; 15: 317–29
Huizing MT, van Warmerdam LJ, Rosing H, et al. Phase I and pharmacologic study of the combination paclitaxel and carboplatin as first-line chemotherapy in stage III and IV ovarian cancer. J Clin Oncol 1997; 15: 1953–64
Grochow LB, Jones RJ, Brundett RB, et al. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol 1989; 25: 55–61
Reece PA, Stafford I, Russell J, et al. Creatinine clearance as a predictor of ultrafilterable platinum disposition in cancer patients treated with cisplatin: relationship between peak ultra-filterable platinum plasma levels and nephrotoxicity. J Clin Oncol 1987; 5: 304–9
Gregg RW, Molepo JM, Monpetit VJ, et al. Cisplatin neurotoxicity: the relationship between dosage, time, and platinum concentration in neurologic tissues, and morphologic evidence of toxicity. J Clin Oncol 1992; 10: 795–803
Santini J, Milano G, Thyss A, et al. 5-FU therapeutic monitoring with dose adjustment leads to an improved therapeutic index in head and neck cancer. Br J Cancer 1989; 59: 287–90
Gupta E, Lestingi TM, Mick R, et al. Metabolic fate of irinotecan in humans: correlation of glucuronidation with diarrhea. Cancer Res 1994; 54: 3723–5
Desai ZR, Van Den Berg HW, Bridges JM, et al. Can severe vincristine neurotoxicity be prevented? Cancer Chemother Pharmacol 1982; 8: 211–4
Reilly JJ, Workman P. Normalisation of anti-cancer drug dosage using body weight and surface area: is it worthwhile? A review of theoretical and practical considerations. Cancer Chemother Pharmacol 1993; 32: 411–8
Cassidy J. Chemotherapy administration: doses, infusions and choice of schedule. Ann Oncol 1994; 5: 25–9; discussion 29–30
Alexander JK, Dennis EW, Smith WG, et al. Blood volume, cardiac output, and distribution of systemic blood flow in extreme obesity. Cardiovasc Res Cent Bull 1962; 1: 39–44
Feldschuh J, Enson Y. Prediction of the normal blood volume: relation of blood volume to body habitus. Circulation 1977; 56: 605–12
Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 1916; 17: 863–71
Gehan EA, George SL. Estimation of human body surface area from height and weight. Cancer Chemother Rep 1970; 54: 225–35
Grochow LB, Baraldi C, Noe D. Is dose normalization to weight or body surface area useful in adults? JNatl Cancer Inst 1990; 82: 323–5
Reilly JJ, Workman P. Is body composition an important variable in the pharmacokinetics of anticancer drugs? A review and suggestions for further research. Cancer Chemother Pharmacol 1994; 34: 3–13
Baker SD, Grochow LB, Donehower RC. Should anticancer drug doses be adjusted in the obese patient? J Natl Cancer Inst 1995; 87: 333–4
Ratain MJ. Body surface area as a basis for dosing of anticancer agents: science, myth or habit? J Clin Oncol 1998; 16: 2297–8
Dobbs NA, Twelves CJ. What is the effect of adjusting epirubicin doses for body surface area. Br J Cancer 1998; 78: 662–6
Gurney HP, Ackland S, Gebski V, et al. Factors affecting epirubicin pharmacokinetics and toxicity: evidence against using body surface area for dose calculation. J Clin Oncol 1998; 16: 2299–304
Gurney H. Dose calculation of anticancer drugs: areview of the current practice and introduction of an alternative. J Clin Oncol 1996; 14: 2590–611
Kintzel PE, Dorr RT. Anticancer drug renal toxicity and elimination: dosing guidelines for altered renal function. Cancer Treat Rev 1995; 21: 33–64
Koren G, Beatty K, Seto A, et al. The effects of impaired liver function on the elimination of antineoplastic agents. Ann Pharmacother 1992; 26: 363–71
Donelli MG, Zucchetti M, Munzone E, et al. Pharmacokinetics of anticancer agents in patients with impaired liver function. Eur J Cancer 1998; 34: 33–46
Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 1989; 7: 1748–56
Chatelut E, Canal P, Brunner V, et al. Prediction of carboplatin clearance from standard morphological and biological patient characteristics. J Natl Cancer Inst 1995; 87: 573–80
Newell DR, Siddik ZH, Gumbrell LA, et al. Plasma free platinum pharmacokinetics in patients treated with high dose carboplatin. Eur J Cancer Clin Oncol 1987; 23: 1399–405
Shea TC, Flaherty M, Elias A, et al. A phase I clinical and pharmacokinetic study of carboplatin and autologous bone marrow support [published erratum appears in J Clin Oncol 1989 Aug; 7 (8): 1177]. J Clin Oncol 1989; 7: 651–1
Mulder PO, de Vries EG, Uges DR, et al. Pharmacokinetics of carboplatin at a dose of 750 mg m-2 divided over three consecutive days. Br J Cancer 1990; 61: 460–4
Calvert AH, Lind MJ, Ghazal Aswad S, et al. Carboplatin and granulocyte colony-stimulating factor as first-line treatment for epithelial ovarian cancer: a phase I dose-intensity escalation study. Semin Oncol 1994; 21: 1–6
Lind MJ, Ghazal Aswad S, Gumbrell L, et al. Phase I study of pharmacologically based dosing of carboplatin with filgrastim support in women with epithelial ovarian cancer. J Clin Oncol 1996; 14: 800–5
Siddiqui N, Boddy AV, Thomas HD, et al. A clinical and pharmacokinetic study of the combination of carboplatin and paclitaxel for epithelial ovarian cancer. Br J Cancer 1997; 75: 287–94
Egorin MJ, Van Echo DA, Olman EA, et al. Prospective validation of a pharmacologically based dosing scheme for the cis-diamminedichloroplatinum(II) analogue diamminecyclo-butanedicarboxylatoplatinum. Cancer Res 1985; 45: 6502–6
Calvert AH, Boddy A, Bailey NP, et al. Carboplatin in combination with paclitaxel in advanced ovarian cancer: dose determination and pharmacokinetic and pharmacodynamic interactions. Semin Oncol 1995; 22 Suppl. 12: 91–8
van Wamerdam LJC, Rodenhuis S, Ten Bokkel Huinink WW, et al. Evaluation of formulas using the serum creatinine level to calculate the optimal dosage of carboplatin. Cancer Chemother Pharmacol 1996; 37: 266–70
Fujiwara Y, Takahashi T, Yamakido M, et al. Re: prediction of carboplatin clearance from standard morphological and biological patient characteristics [comment on: J Natl Cancer Inst 1995 Apr 19; 87 (8): 573-80]. J Natl Cancer Inst 1997; 89: 260–2
Minami H, Ando Y, Saka H, et al. Re: prediction of carboplatin clearance from standard morphological and biological patient characteristics [comment on: J Natl Cancer Inst 1997 Feb 5; 89 (3): 260-2]. J Natl Cancer Inst 1997; 89: 968–70
Kearns CM, Egorin MJ. Considerations regarding the less-than-expected thrombocytopenia encountered with combination paclitaxel/carboplatin chemotherapy. Semin Oncol 1997; 24 (1 Suppl. 2): S2–91–6
Calvert AH. A review of the pharmacokinetics and pharmaco-dynamics of combination carboplatin/paclitaxel. Semin Oncol 1997; 24 (1 Suppl. 2): S2–85–90
Alberts DS, Garcia DJ. Total platinum dose versus platinum dose intensification in ovarian cancer treatment. Semin Oncol 1994; 21: 11–5
Okamoto H, Nagatomo A, Kunitoh H, et al. The predictive performance of carboplatin clearance by patient characteristics or 24-hour creatinine clearance. Proc Am Assoc Clin Oncol 1996; 15: 174
Hande KR. The importance of drug scheduling in cancer chemotherapy: etoposide as an example. Stem Cells 1996; 14: 18–24
Miller AA, Stewart CF, Tolley EA. Clinical pharmacodynamics of continuous-infusion etoposide. Cancer Chemother Pharmacol 1990; 25: 361–6
Mick R, Ratain MJ. Modeling interpatient pharmacodynamic variability of etoposide. J Natl Cancer Inst 1991; 83: 1560–4
Joel SP, Shah R, Clark PI, et al. Predicting etoposide toxicity: relationship to organ function and protein binding. J Clin Oncol 1996; 14: 257–67
Nguyen L, Chatelut E, Chevreau C, et al. Population pharmacokinetics of total and unbound etoposide. Cancer Chemother Pharmacol 1998; 41: 125–32
Joel SP, Hall M, Gaver RC, et al. Complete recovery of radioactivity after administration of 14C-etoposide in man. Proc Am Assoc Clin Oncol 1995; 14: 168
Stewart CF, Fleming RA, Arbuck SG, et al. Prospective evaluation of a model for predicting etoposide plasma protein binding in cancer patients. Cancer Res 1990; 50: 6854–6
Stewart CF, Arbuck SG, Fleming RA, et al. Changes in the clearance of total and unbound etoposide in patients with liver dysfunction. J Clin Oncol 1990; 8: 1874–9
Stewart CF, Pieper JA, Arbuck SG, et al. Altered protein binding of etoposide in patients with cancer. Clin Pharmacol Ther 1989; 45: 49–55
Arbuck SG, Douglass HO, Crom WR, et al. Etoposide pharmacokinetics in patients with normal and abnormal organ function. J Clin Oncol 1986; 4: 1690–5
Sonnichsen DS, Hurwitz CA, Pratt CB, et al. Saturable pharmacokinetics and paclitaxel pharmacodynamics in children with solid tumors. J Clin Oncol 1994; 12: 532–8
Sonnichsen DS, Relling MV. Clinical pharmacokinetics of paclitaxel. Clin Pharmacokinet 1994; 27: 256–69
Gianni L, Kearns CM, Giani A, et al. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. J Clin Oncol 1995; 13: 180–90
Sonnichsen DS, Liu Q, Schuetz EG, et al. Variability in human cytochrome P450 paclitaxel metabolism. J Pharmacol Exp Ther 1995; 275: 566–75
Venook AP, Egorin M, Rosner TD, et al. Phase I and pharmacokinetic trial of paclitaxel in patients with hepatic dysfunction: Cancer and Leukemia Group B 9264. J Clin Oncol 1994; 13: 1811–9
Huizing MT, Rosing H, Vermoken JB. Pharmacology of paclitaxel and its metabolites in patients with altered liver function. Eur J Cancer 1995; 31A Suppl. 5: S192
Nannan Panday VR, Huizing MT, Willemse PHB, et al. Hepatic metabolism of paclitaxel and its impact in patients with altered hepatic function. Semin Oncol 1997; 24 Suppl.: S11–34–8
Bruno R, Sanderink GJ. Pharmacokinetics and metabolism of taxotere (docetaxel). Cancer Surv 1993; 17: 305–13
Launay Iliadis MC, Bruno R, Cosson V, et al. Population pharmacokinetics of docetaxel during phase I studies using nonlinear mixed-effect modeling and nonparametric maximum-likelihood estimation. Cancer Chemother Pharmacol 1995; 37: 47–54
Robert J, Gianni L. Pharmacokinetics and metabolism of anthracyclines. Cancer Surv 1993; 17: 219–52
Robert J. Epirubicin: clinical pharmacology and dose-effect relationship. Drugs 1993; 45 Suppl. 2: 20–30
Robert J. Clinical pharmacokinetics of epirubicin. Clin Pharmacokinet 1994; 26: 428–38
Reich SD. Clinical correlation of adriamycin pharmacology. Pharmacol Ther 1978; 5: 304–9
Twelves CJ, Dobbs NA, Gillies HC, et al. Doxorubicin pharmacokinetics: the effect of abnormal liver biochemistry tests. Cancer Chemother Pharmacol 1998; 42: 229–34
Twelves CJ, Dobbs NA, Michael Y, et al. Clinical pharmacokinetics of epirubicin: the importance of liver biochemistry tests. Br J Cancer 1992; 66: 765–9
Verweij J, Lund B, Beijnen J, et al. Phase I and pharmacokinetics study of topotecan, a new topoisomerase I inhibitor. Ann Oncol 1993; 4: 673–8
O’Dwyer P, LaCreta FP, Haas NB, et al. Clinical, pharmacokinetic and biological studies of topotecan. Cancer Chemother Pharmacol 1994; 34: S46–52
Dennis MJ, Beijnen JH, Grochow LB, et al. An overview of the clinical pharmacology of topotecan. Semin Oncol 1997; 24
O’Reilly S, Rowinsky EK, Slichenmyer W, et al. Phase I and pharmacologic study of topotecan in patients with impaired renal function. J Clin Oncol 1996; 14: 3062–73
O’Reilly S, Rowinsky E, Slichenmyer W, et al. Phase I and pharmacologic studies of topotecan in patients with impaired hepatic function. J Natl Cancer Inst 1996; 88: 817–24
Lind MJ, Ardiet C. Pharmacokinetics of alkylating agents. Cancer Surv 1993; 17: 157–88
Kaijser GP, Beijnen JH, Bult A, et al. Ifosfamide metabolism and pharmacokinetics. Anticancer Res 1994; 14: 517–31
Boddy AV, Proctor M, Simmonds D, et al. Pharmacokinetics, metabolism and clinical effect of ifosfamide in breast cancer patients. Eur J Cancer 1995; 31A: 69–76
Busse D, Busch FW, Bohnenstengel F, et al. Dose escalation of cyclophosphamide in patients with breast cancer: consequences for pharmacokinetics and metabolism. J Clin Oncol 1997; 15: 1885–96
Chen TL, Kennedy MJ, Anderson LW, et al. Nonlinear pharmacokinetics of cyclophosphamide and 4-hydroxycyclophos-phamide/aldophosphamide in patients with metastatic breast cancer receiving high-dose chemotherapy followed by autologous bone marrow transplantation. Drug Metab Disp 1997; 25: 544–1
Wagner T. Ifosfamide clinical pharmacokinetics. Clin Pharmacokinet 1994; 26: 439–56
Rahmani R, Zhou XJ. Pharmacokinetics and metabolism of vinca alkaloids. Cancer Surv 1993; 17: 269–81
Van Den Berg HW, Desai ZR, Wilson R, et al. The pharmacokinetics of vincristine in man: reduced drug clearance associated with raised serum alkaline phosphatase and dose-limited elimination. Cancer Chemother Pharmacol 1982; 8: 215–9
Leveque D, Jehl F. Clinical pharmacokinetics of vinorelbine. Clin Pharmacokinet 1996; 31: 184–97
Robieux I, Sorio R, Borsatti E, et al. Pharmacokinetics of vinorelbine in patients with liver metastases. Clin Pharmacol Ther 1996; 59: 32–40
Pinguet F, Martel P, Fabbro M, et al. Pharmacokinetics of high-dose intravenous melphalan in patients undergoing peripheral blood hematopoietic progenitor-cell transplantation. Anticancer Res 1997; 17: 605–11
Osterborg A, Ehrsson H, Eksborg S, et al. Pharmacokinetics of oral melphalan in relation to renal function in multiple myeloma patients. Eur J Cancer Clin Oncol 1989; 25: 899–903
Kergueris MF, Milpied N, Moreau P, et al. Pharmacokinetics of high-dose melphalan in adults: influence of renal function. Anticancer Res 1994; 14: 2379–82
Baker SD, Grochow LB. Pharmacology of cancer chemotherapy in the older person. Clin Geriatr Med 1997; 13: 169–83
Egorin MJ. Cancer pharmacology in the elderly. Semin Oncol 1993; 20: 43–9
Etienne MC, Chatelut E, Pivot X, et al. Co-variables influencing 5-fluorouracil clearance during continuous venous infusion: a NONMEM analysis. Eur J Cancer 1998; 34: 92–7
Gelman RS, Taylor SG. Cyclophosphamide, methotrexate, and 5-fluorouracil chemotherapy in women more than 65 years old with advanced breast cancer: the elimination of age trends in toxicity by using doses based on creatinine clearance. J Clin Oncol 1984; 2: 1404–3
Borkowski JM, Duerr M, Donehower RC, et al. Relation between age and clearance rate of nine investigational anti-cancer drugs from phase I pharmacokinetic data. Cancer Chemother Pharmacol 1994; 33: 493–6
Bonetti A, Franceschi T, Apostoli P, et al. Cisplatin pharmacokinetics in elderly patients. Ther Drug Monit 1994; 16: 477–82
Yamamoto N, Tamura T, Maeda M, et al. The influence of ageing on cisplatin pharmacokinetics in lung cancer patients with normal organ function. Cancer Chemother Pharmacol 1995; 36: 102–6
Sorio R, Robieux I, Galligioni E, et al. Pharmacokinetics and tolerance of vinorelbine in elderly patients with metastatic breast cancer. Eur J Cancer 1997; 33: 301–3
Desoize B, Robert J. Individual dose adaptation of anticancer drugs. Eur J Cancer 1994; 30A(6): 844–51
Gelman RS, Tormey DC, Betensky R, et al. Actual versus ideal weight in the calculation of surface area: effects on dose of 11 chemotherapy agents. Cancer Treat Rep 1987; 71: 907–11
Georgiadis MS, Steinberg SM, Hankins LA, et al. Obesity and therapy-related toxicity in patients treated for small-cell lung cancer. J Natl Cancer Inst 1995; 87: 361–6
Powis G, Reece P, Ahman DL, et al. Effect of body weight on the pharmacokinetics of cyclophosphamide in breast cancer patients. Cancer Chemother Pharmacol 1987; 20: 219–22
Rodvold KA, Rushing DA, Tewksbury DA. Doxorubicin clearance in the obese. J Clin Oncol 1988; 6: 1321–7
Lind MJ, Margison JM, Cerny T, et al. Prolongation of ifosfamide elimination half-life in obese patients due to altered drug distribution. Cancer Chemother Pharmacol 1989; 25: 139–42
Benezet S, Guimbaud R, Chatelut E, et al. How to predict carboplatin clearance from standard morphological and biological characteristics in obese patients. Ann Oncol 1997; 8: 607–9
Houyau P, Gay C, Chatelut E, et al. Severe fluorouracil toxicity in a patient with dihydropyrimidine dehydrogenase deficiency [letter]. J Natl Cancer Inst 1993; 85: 1602–3
Meinsma R, Fernandez-Salguero P, van Kulenberg AB, et al. Human polymorphism in drug metabolism: mutation in the dihydropyrimidine dehydrogenase gene results in exon skipping and thymine uracilurea. DNA Cell Biol 1995; 14: 1–6
Takai S, Fernandez-Salguero P, Kimura S, et al. Assignment of the human dihydropymidine dehydrogenase gene to chromosome region 1p22 by fluorescence in situ hybridisation. Genomics 1994; 24: 613–4
Albin N, Jonhson MR, Shahinian H, et al. Initial characterization of the molecular defect in human dihydropyrimidine dehydrogenase deficiency. Proc Am Assoc Cancer Res 1995; 36: 211
Fleming RA, Milano G, Thyss A, et al. Correlation between dihydropyrimidine dehydrogenase activity in peripheral mononuclear cells ans systemic clearance of fluorouracil in cancer patients. Cancer Res 1992; 52: 2899–902
Etienne MC, Lagrange JL, Dassonville O, et al. Population study of dihydropyrimidine dehydrogenase in cancer patients [see comments]. J Clin Oncol 1994; 12: 2248–53
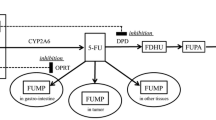
Milano G, Etienne MC. Potential importance of dihydropyrimidine dehydrogenase (DPD) in cancer chemotherapy. Pharmacogenetics 1994; 4: 301–6
Lennard L. The clinical pharmacology of 6-mercaptopurine. Eur J Clin Pharmacol 1992; 43: 329–9
Lennard L, Lilleyman JS, Van Loon J, et al. Genetic variation in response to 6-mercaptopurine for childhood acute lympho-blastic leukaemia. Lancet 1990; 336: 225–9
Tinel M, Berson A, Pessayre D, et al. Pharmacogenetics of human erythrocyte thiopurine methyltransferase activity in a French population. Br J Clin Pharmacol 1991; 32: 729–34
Lennard L, Van Loon JA, Lilleyman JS, et al. Thiopurine pharmacogenetics in leukemia: correlation of erythrocyte thiopurine methyltransferase activity and 6-thioguanine nucleotide concentrations. Clin Pharmacol Ther 1987; 41: 18–25
Evans WE, Horner M, Chu YQ, et al. Altered mercaptopurine metabolism, toxic effects, and dosage requirement in a thiopurine methyltransferase-deficient child with acute lymphocytic leukemia. J Pediatr 1991; 119: 985–9
Klemetsdal B, Tollefsen E, Loennechen T, et al. Interethnic difference in thiopurine methyltransferase activity. Clin Pharmacol Ther 1992; 51: 24–31
Cresteil T, Monsarrat B, Alvinerie P, et al. Taxol metabolism by human liver microsomes: identification of cytochrome P450 isoenzymes involved in its biotransformation. Cancer Res 1994; 54: 386–92
Royer I, Monsarrat B, Sonnier M, et al. Metabolism of docetaxel by human cytochromes P450: interactions with paclitaxel and other antineoplastic drugs. Cancer Res 1996; 56: 58–65
Haaz MC, Rivory LP, Riche C, et al. The transformation of irinotecan (CPT-11) to its active metabolite SN-38 by human liver microsomes. Differential hydrolysis for the lactone and carboxylate forms. Naunyn Schmiedebergs Arch Pharmacol 1997; 356: 257–62
Relling MV, Nemec J, Schuetz EG, et al. O-demethylation of epipodophyllotoxin is catalyzed by human cytochrome P450 3A4. Mol Pharmacol 1994; 45: 352–8
Kivisto KT, Kroemer HK, Eichelbaum M. The role of human cytochrome P450 enzymes in the metabolism of anticancer agents: implications for drug interactions. Br J Clin Pharmacol 1995; 40: 523–30
Chang SM, Kuhn JG, Rizzo J, et al. Phase I study of paclitaxel in patients with recurrent malignant glioma: a North American Brain Tumour consortium report. J Clin Oncol 1998; 16: 188–84
Mackenzie PI, Owens IS, Burchell B, et al. The UDP glycosyltransferase gene superfamily: recommended nomenclature update based on evolutionary divergence. Pharmacogenetics 1997; 7: 255–69
Chabot GG. Clinical pharmacokinetics of irinotecan. Clin Pharmacokinet 1997; 33: 245–59
Iyer L, King CD, Whitington PR, et al. Genetic predisposition to the metabolism of irinotecan (CPT-11): Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J Clin Invest 1998; 101: 847–54
Wasserman E, Myara A, Lokiec F, et al. Severe CPT-11 toxicity in patients with Gilbert’s syndrome: two case reports. J Clin Invest 1997; 8: 1049–51
Ando Y, Saka H, Asai G, et al. UGT1A1 genotypes and glucuronidation of SN-38, the active metabolite of irinotecan. Ann Oncol 1998; 9: 845–7
Favre R, Monjanel S, Alfonsi M, et al. High-dose methotrexate: a clinical and pharmacokinetic evaluation. Cancer Chemother Pharmacol 1982; 9: 156–60
Thyss A, Schneider M, Santini J, et al. Induction chemotherapy with cis-platinum and 5-fluorouracil for squamous cell carcinoma of the head and neck. Br J Cancer 1986; 54: 755–60
Hillcoat BL, McCulloch PB, Figueredo AT, et al. Clinical response and plasma levels of 5-fluorouracil in patients with colonic cancer treated by drug infusion. Br J Cancer 1978; 38: 719–24
Gamelin EC, Danquechin-Dorval EM, Dumesnil YF, et al. Relationship between 5-fluorouracil (5-FU) dose intensity and therapeutic response in patients with advanced colorectal cancer receiving infusional therapy containing 5-FU. Cancer 1996; 77: 441–51
Gamelin EC, Boisdron-Celle M, Delva RG, et al. Long-term weekly treatment of colorectal metastatic cancer with fluorouracil and leucovorin: results of a multicentric prospective trial of fluorouracil dosage optimization by pharmacokinetic monitoring in 152 patients. J Clin Oncol 1998; 16: 1470–8
Clark PI, Slevin ML. The clinical pharmacology of etoposide and teniposide. Clin Pharmacokinet 1987; 12: 223–52
Slevin ML, Clark PL, Joel SP, et al. A randomized trial to evaluate the effect of schedule on the activity of etoposide in small-cell lung cancer. J Clin Oncol 1989; 7: 1333–40
Ratain MJ, Mick R, Schilsky RL, et al. Pharmacologically based dosing of etoposide: a means of safely increasing dose intensity. J Clin Oncol 1991; 9: 1480–6
Kunitoh H, Watanabe K. Phase I/II and pharmacologic study of long-term continuous infusion etoposide combined with cisplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol 1994; 12: 83–9
Miller AA, Herndon JE, Hollis DR, et al. Schedule dependency of 21-day oral versus 3-day intravenous etoposide in combination with intravenous cisplatin in extensive-stage small-cell lung cancer: a randomized phase III study of the Cancer and Leukemia Group B. J Clin Oncol 1995; 13: 1871–9
Joel SP, Clark PI, Heap L, et al. Pharmacological attempts to improve the bioavailability of oral etoposide. Cancer Chemother Pharmacol 1995; 37: 125–33
Sessa C, Zucchetti M, Torri V, et al. Chronic oral etoposide in small-cell lung cancer: clinical and pharmacokinetic results. Ann Oncol 1993; 4: 553–8
Nirenberg A, Mosende C, Mehta BM, et al. High-dose methotrexate with citrovorum factor rescue: predictive value of serum methotrexate concentrations and corrective measures to avert toxicity. Cancer Treat Rep 1977; 61: 779–83
Wolfrom C, Hepp R, Hartmann R, et al. Pharmacokinetic study of methotrexate, folinic acid and their serum metabolites in children treated with high-dose methotrexate and leucovorin rescue. Eur J Clin Pharmacol 1990; 39: 377–83
Pearson AD, Amineddine HA, Yule M, et al. The influence of serum methotrexate concentrations and drug dosage on outcome in childhood acute lymphoblastic leukaemia. Br J Cancer 1991; 64: 169–73
Stoller RG, Hande KR, Jacobs SA, et al. Use of plasma pharmacokinetics to predict and prevent methotrexate toxicity. N Engl J Med 1977; 297: 630–4
Borsi JD, Moe PJ. Systemic clearance of methotrexate in the prognosis of acute lymphoblastic leukemia in children. Cancer 1987; 60: 3020–4
Camilla B, Mahoney D, Leventhal B, et al. Intensive intravenous methotrexate and mercaptopurine treatment of higherrisk non-T, non-B acute lymphocytic leukemia: a pediatric oncology group study. J Clin Oncol 1994; 12: 1383–9
Whitehead VM, Vuchich MJ, Lauer SJ, et al. Accumulation of high levels of methotrexate polyglutamates in lymphoblasts from children with hyperdiploid (greater than 50 chromosomes) B-lineage acute lymphoblastic leukemia: a Pediatrie Oncology Group study. Blood 1992; 80: 1316–23
Chabner BA, Allegra CJ, Curt GA, et al. Polyglutamation of methotrexate: is methotrexate a prodrug? J Clin Invest 1985; 76: 907–12
Grochow LB. Busulfan disposition: the role of therapeutic monitoring in bone marrow transplantation induction regimens. Semin Oncol 1993; 20: 18–25
Schuler U, Schroer S, Kuhnle A, et al. Busulfan pharmacokinetics in bone marrow transplant patients: is drug monitoring warranted? Bone Marrow Transplant 1994; 14: 759–65
Dix SP, Wingard JR, Mullins RE, et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant 1996; 17: 225–30
Slattery JT, Clift RA, Buckner CD, et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood 1997; 89: 3055–60
Yeager AM, Wagner Jr JE, Graham ML, et al. Optimization of busulfan dosage in children undergoing bone marrow transplantation: a pharmacokinetic study of dose escalation. Blood 1992; 80: 2425–8
Vassal G, Fischer A, Challine D, et al. Busulfan disposition below the age of three: alteration in children with lysosomal storage disease. Blood 1993; 82: 1030–4
Shaw PJ, Scharping CE, Brian RJ, et al. Busulfan pharmacokinetics using a single daily high-dose regimen in children with acute leukemia. Blood 1994; 84: 2357–62
Hassan M, Ehrsson H, Ljungman P. Aspects concerning busulfan pharmacokinetics and bioavailability. Leukemia Lymphoma 1996; 22: 395–407
Hassan M, Fasth A, Gerritsen B, et al. Busulphan kinetics and limited sampling model in children with leukemia and inherited disorders. Bone Marrow Transplant 1996; 18: 843–50
Vassal G, Koscielny S, Challine D, et al. Busulfan disposition and hepatic veno-occlusive disease in children undergoing bone marrow transplantation. Cancer Chemother Pharmacol 1996; 37: 247–53
Judson IR. Pharmacokinetic modelling —a prelude to therapeutic drug monitoring for all cancer patients? Eur J Cancer 1995; 31A: 1733–5
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Canal, P., Chatelut, E. & Guichard, S. Practical Treatment Guide for Dose Individualisation in Cancer Chemotherapy. Drugs 56, 1019–1038 (1998). https://doi.org/10.2165/00003495-199856060-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199856060-00006