Abstract
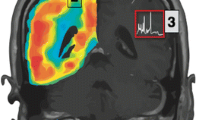
We examined in the present investigation regional ATP, glucose, and lactate content in the cortical and subcortical structures, in a rat model of closed head injury (CHI). In serial tissue sections bioluminescence imaging of ATP, glucose, and lactate was performed at 4 h, 12 h and 24 h (n=4/5 per time point with) after the induction of CHI or sham surgery. Bioluminescence images were analyzed by computer-assisted densitometry, at the lesion site, in remote cortical areas, and in the subcortical structures (thalamus and caudate nucleus). ATP content was significantly decreased at the lesion site after 4 h and in the remote cortex at 12 h post-injury. At 12 h, the ATP content reached baseline levels on the ipsilateral side and at 24 h also at remote lateral parietal sites. In the contralateral cortex, ATP increased transiently above the baseline at 12 h. No significant changes in ATP were found in the thalamus and caudate nucleus. Cortical glucose and lactate contents could not be discerned over time.
Following CHI there is an acute and progressive, yet transient, ischemic cortical profile, which is not reflected in subcortical areas.
Similar content being viewed by others
References
Armstead W. M. (1999) Age-dependent impairment of K(ATP) channel function following brain injury. J. Neurotrauma 16(5), 391–402.
Assaf Y., Holokovsky A., Bermann E., Shapira Y., Shohami E., and Cohen Y. (1999) Diffusion and perfusion MRI following closed head injury in rats. J. Neurotrauma 16, 1165–1176.
Azbill R., Mu X., Bruce-Keller A. J., Mattson M. P., and Springer J. (1997) Impaired mitochondrial function, oxidative stress and altered antioxidant enzyme activities following traumatic spinal cord injury. Brain Res. 765, 283–290.
Barone F. C., Ohlstein E. H., Hunter A. J., et al. (2000) Selective antagonism of ETA receptors improves outcome in both head trauma and focal stroke. J. Cardiovasc. Pharmacol. 36, 357–361.
Beit-Yannai E., Kohen R., Horowitz M., Trembovler V., and Shohami E. (1997) Changes in biological reducing activity in rat brain following closed head injury: a cyclic voltammetry study in normal and acclimated rats. J. Cereb. Blood Flow Metabol. 17, 273–279.
Bell M. J., Kochanek P. M., Doughty L. A., Carcillo J. A., Adelson P. D., Clark R. S., et al. (1997) Interleukin-6 and interleukin-10 in cerebrospinal fluid after severe traumatic brain injury in children. J. Neurotrauma 14, 451–457.
Bramlett H. M., Kraydieh S., Green E. J., and Dietrich W. D. (1997) Temporal and regional patterns of axonal damage following traumatic brain injury: a beta-amyloid precursor protein immunocytochemical study in rats. J. Neuropathol. Exp. Neurol. 56, 1132–1141.
Fan L., Young P. R., Barone F. C., Feuerstein G. Z., Smith D. H., and McIntosh T. K. (1996) Experimental brain injury induces differential expression of tumor necrosis factor-mRNA in the CNS. Mol. Brain Res. 36, 287–291.
Holmin S., Schalling M., Hojeberg B., Nordqvist A. C., Skeftruna A. K., and Mathiesen T. (1997) Delayed cytokine expression in rat brain following experimental contusion. J. Neurosurg. 86, 493–504.
Kossmann T., Hans V. H. J., Imhof H. G., Stocker R., Grob P., Trentz O., and Morganti-Kossmann M. C. (1994) Intrathecal and serum interleukin-6 and the acute phase response in patients with severe traumatic brain injuries. Shock 4, 311–317.
Krishnappa I. K., Contant C. F., and Robertson C. S. (1999) Regional changes in cerebral extracellular glucose and lactate concentrations following severe cortical impact injury and secondary ischemia in rats. J. Neurotrauma 16, 213–224.
Laurer H. L., Lenzlinger P. M., and McIntosh T. K. (2000) Models of traumatic brain injury. Eur. J. Trauma 26, 95–110.
Lee S. M., Wong M. D., Samii A., and Hovda D. A. (1999) Evidence for energy failure following irreversible traumatic brain injury. Ann. NY Acad. Sci. 893, 337–340.
Marion D. W., Darby J., and Jonas H. (1991) Acute general regional cerebral blood flow changes caused by severe head injuries. J. Neurosurg. 74, 407–414.
Mautes A. E. M., Schröck H., Nacimiento A. C., and Paschen W. (2000) Regional spinal cord blood flow and energy metabolism in rats after laminectomy and acute compression injury. Eur. J. Trauma 26, 122–130.
Meier-Ruge W. A., and Bertoni-Freddari C. (1997) Pathogenesis of decreased glucose turnover and oxidative phosphorylation in ischemic and trauma-induced dementia of the Alzheimer type. Ann. NY Acad. Sci. 826, 229–241.
Murakami N., Yamaki T., Iwamoto Y., Sakakibara T., Kobori N., Fushiki S., and Ueda S. (1998) Experimental brain injury induces expression of amyloid precursor protein, which may be related to neuronal loss in the hippocampus. J. Neurotrauma 15, 993–1003.
Ott L., McClain C. J., Gillespie M., and Young B. (1994) Cytokines and metabolic dysfunction after severe head injury. J. Neurotrauma 11, 447–472.
Pierce J. E., Trojanowski J. Q., Graham D. I., Smith D. H., and McIntosh T. K. (1996) Immunohistochemical characterization of alterations in the distribution of amyloid precursor proteins and beta-amyloid peptide after experimental brain injury in the rat. J. Neurosci. 16, 1083–1090.
Shapira Y., Shohami E., Sidi A., Soffer D., Freeman S., and Cotev S. (1988) Experimental closed head injury in rats: mechanical, pathophysiologic, and neurologic properties. Crit. Care Med. 16, 258–265.
Shohami E., Novikov M., Bass R., Yamin A., and Gallily R. (1994) Closed head injury triggers early production of TNF and IL-6 by brain tissue. J. Cereb. Blood Flow Metabol. 14, 615–619.
Shohami E., Bass R., Wallach D., Yamin A., and Gallily R. (1996) Inhibition of Tumor Necrosis factor activity in rat brain is associated with cerebroprotection after closed head injury. J. Cereb. Blood Flow Metabol. 16, 378–384.
Shohami E., Gallily R., Mechoulam R., Bass R., and Ben-Hur T. (1997) Cytokine production in the brain following closed head injury: Dexanabinol (HU-211) is a novel TNF inhibitor and an effective neuroprotectant. J. Neuroimmunol. 72, 169–177.
Sullivan P. G., Keller J. N., Mattson M. P., and Scheff S. W. (1998) Traumatic brain injury alters synaptic homeostasis: implications for impaired mitochondrial and transport function. J. Neurotrauma 15, 789–798.
Vagnozzi R., Marmarou A., Tavazzi B., Signoretti S., Di Pierro D., del Bolgia F., et al. (1999) Changes of energy metabolism and lipid peroxidation in rats leading to mitochondrial dysfunction after diffuse brain injury. J. Neurotrauma 16, 903–913.
Woodroofe M. N., Sarna G. S., Wadhwa M., Hayes G. M., Loughlin A. J., Tiner A., and Cuzner M. L. (1991) Detection of interleukin-1 and interleukin-6 in adult rat brain following mechanical injury, by in vivo microdialysis: evidence of a role for microglia in cytokine production. J. Neuroimmunol. 33, 227–236.
Yoshino A., Hovda D. A., Kawamata T., Katayama Y., and Becker D. P. (1991) Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res. 561, 106–119.
Yu N., Maciejewski-Lenoir D., Bloom F. E., and Magistretti P. J. (1995) Tumor necrosis factor-alpha and interleukin-1 alpha enhance glucose utilization by astrocytes: involvement of phospholipase A2. Mol. Pharmacol. 48, 550–558.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mautes, A.E.M., Thome, D., Steudel, WI. et al. Changes in regional energy metabolism after closed head injury in the rat. J Mol Neurosci 16, 33–39 (2001). https://doi.org/10.1385/JMN:16:1:33
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:16:1:33