Abstract
Purpose
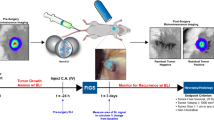
A complete surgical excision with negative tumor margins is the single most important factor in the prediction of long-term survival for most cancer patients with solid tumors. We hypothesized that image-guided surgery using nanoparticle-enhanced photoacoustic and fluorescence imaging could significantly reduce the rate of local recurrence.
Methods
A murine model of invasive mammary carcinoma was utilized. Three experimental groups were included: (1) control; (2) tumor-bearing mice injected with non-targeted nanoprobe; and (3) tumor-bearing mice injected with targeted nanoprobe. The surgeon removed the primary tumor following the guidance of photoacoustic imaging (PAI), then inspected the surgical wound and removed the suspicious tissue using intraoperative near-infrared (NIR) fluorescence imaging. The mice were followed with bioluminescence imaging weekly to quantify local recurrence.
Results
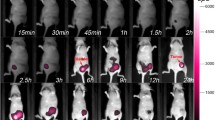
Nanoprobe-enhanced photoacoustic contrast enabled PAI to map the volumetric tumor margins up to a depth of 31 mm. The targeted nanoparticles provided significantly greater enhancement than non-targeted nanoparticles. Seven mice in the group injected with the targeted nanoprobes underwent additional resections based upon NIR fluorescence imaging. Pathological analysis confirmed residual cancer cells in the re-resected specimens in 5/7 mice. Image-guided resection resulted in a significant reduction in local recurrence; 8.7 and 33.3 % of the mice in the targeted and control groups suffered recurrence, respectively.
Conclusions
These results suggest that photoacoustic and NIR intraoperative imaging can effectively assist a surgeon to locate primary tumors and to identify residual disease in real-time. This technology has promise to overcome current clinical challenges that result in the need for second surgical procedures.




Similar content being viewed by others
References
Aliperti LA, Predina JD, Vachani A, Singhal S. Local and systemic recurrence is the Achilles heel of cancer surgery. Ann Surg Oncol. 2011;18:603–607.
Taghian A, Mohiuddin M, Jagsi R, Goldberg S, Ceilley E, Powell S. Current perceptions regarding surgical margin status after breast-conserving therapy: results of a survey. Ann Surg. 2005;241(4):629–39.
Assersohn L, Powles TJ, Ashley S, et al. Local relapse in primary breast cancer patients with unexcised positive surgical margins after lumpectomy, radiotherapy and chemoendocrine therapy. Ann Oncol. 1999;10(12):1451–5.
Balch GC, Mithani SK, Simpson JF, Kelley MC. Accuracy of intraoperative gross examination of surgical margin status in women undergoing partial mastectomy for breast malignancy. Am Surg. 2005;71(1):22–7.
Sigal-Zafrani B, Lewis JS, Clough KB, et al. Histological margin assessment for breast ductal carcinoma in situ: precision and implications. Mod Pathol. 2004;17(1):81–8.
Abraham SC, Fox K, Fraker D, Solin L, Reynolds C. Sampling of grossly benign breast reexcisions: a multidisciplinary approach to assessing adequacy. Am J Surg Pathol. 1999;23:316–22.
Marmulla R, Hilbert M, Niedert-Ellmann H. Introperative precision of mechanical, electromechanical, infrared and laser-guided navigation systems in computer-assisted surgery. Mund Kiefer Gesichtschir. 1998;2:145–8.
Hassfeld S, Muhling J. Comparative examination of the accuracy of a mechanical and an optical system in CT and MRT based instrument navigation. Int J Oral Maxillofac Surg. 2000;29(6):400–7.
Wagner A, Schicho K, Birkfellner W, et al. Quantitative analysis of factors affecting intraoperative precision and stability of optoelectronic and electromagnetic tracking systems. Med Phys. 2002;29(5):905–12.
Bigio IJ, Bown SG, Briggs G, et al. Diagnosis of breast cancer using elastic-scattering spectroscopy: preliminary clinical results. J Biomed Opt. 2000;5(2): 221–8.
Haka AS, Volynskaya Z, Gardecki JA, et al. In vivo margin assessment during partial mastectomy breast surgery using Raman spectroscopy. Cancer Res. 2006;66(6):3317–22.
Kennedy S, Geradts J, Bydlon T, et al. Optical breast cancer margin assessment: an observational study of the effects of tissue heterogeneity on optical contrast. Breast Cancer Res. 2010;12(6): R91.
Nguyen FT, Zysk AM, Chaney EJ, et al. Intraoperative evaluation of breast tumor margins with optical coherence tomography. Cancer Res. 2009;69(22):8790–6.
Yang H, Xi L, Samuelson S, Xie H, Yang L, Jiang H. Handheld miniature probe integrating diffuse optical tomography with photoacoustic imaging through a MEMS scanning mirror. Biomed Opt Express. 2013;4: 427–32.
He B, Xi L, Samuelson SR, Xie H, Yang L, Jiang H. Microelectromechanical systems scanning-mirror-based handheld probe for fluorescence molecular tomography. Appl Opt. 2012;51:4678–83.
Wang LV. Multiscale photoacoustic microscopy and computed tomography. Nat Photonics. 2009;3(9):503–9.
Beard P. Biomedical photoacoustic imaging. Interface Focus. 2011;1:602–31.
Xi L, Sun J, Zhu Y, Wu L, Xie H, Jiang H. Photoacoustic imaging based on MEMS mirror scanning. Biomed Opt Express. 2010;1:1278–83.
Xi L, Grobmyer SR, Wu L, et al. Evaluation of breast tumor margins in vivo with intraoperative photoacoustic imaging. Opt Express. 2012;20:8726–31.
Pan D, Pramanik M, Senpan A, et al. Molecular photoacoustic imaging of angiogenesis with integrin targeted gold nanobeacons. FASEB J. 2011;25(3):875–82.
Kim C, Cho EC, Chen J, et al. In vivo molecular photoacoustic tomography of melanomas targeted by bioconjugated gold nanocages. ACS Nano. 2010;4:4559–64.
Pan D, Pramanik M, Senpan A, Ghosh S, Wickline SA, Wang LV, et al. Near infrared photoacoustic detection of sentinel lymph nodes with gold nanobeacons. Biomaterials. 2010;31:4088–93.
Kim C, Song KH, Gao F, Wang LV. Sentinel lymph nodes and lymphatic vessels: noninvasive dual-modality in vivo mapping by using indocyanine green in rats-volumetric spectroscopic photoacoustic imaging and planar fluorescence imaging. Radiology. 2010;255: 442–50.
Yang XM, Skrabalak SE, Li Z, Xia Y, Wang LV. Photoacoustic tomography of a rat cerebral cortex in vivo with au nanocages as an optical contrast agent. Nano Lett. 2007;7 (12):3798–802.
Yang L, Peng XH, Wang A, et al. Receptor-targeted nanoparticles for in vivo imaging of breast cancer. Clin Cancer Res. 2009;15:4722–32.
Xi L, Grobmyer SR, Zhou G, Qian W, Yang L, Jiang H. Molecular photoacoustic tomography of breast cancer using receptor targeted magnetic iron oxide nanoparticles as contrast agents. J Biophotonics. doi:10.1002/Jbio00155.
De Grand AM, Frangioni JV. An operational near-infrared fluorescence imaging system prototype for large animal surgery. Technol Cancer Res Treat. 2003;2:553–62.
Tanaka E, Choi HS, Fujii H, Bawerndi MG, Frangion JV. Image-guided oncologic surgery using invisible light: completed pre-clinical development for sentinel lymph node mapping. Ann Surg Oncol. 2006;13:1671–81.
Troyan SL, Kianzad V, Gibbs-Strauss SL, et al. The FLARE™ intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol. 2009;16:2943–52.
Gioux S, Kianzad V, Ciocan R, Gupta S, Oketokoun R, Frangion JV. High-power, computer-controlled, light-emitting diode-based light sources for fluorescence imaging and image-guided surgery. Mol Imaging. 2009;8(3):156–65.
Van Dam GM, Themelis G, Crane LM, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat Med. 2011;17:1315–9.
Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348(25):2491–9.
Satpathy M, Wang L, Zielinski R, et al. Active targeting using HER-2-affibody-conjugated nanoparticles enabled sensitive and specific imaging of orthotopic HER-2 positive ovarian tumors. Small. 2013;10(3):544–55. doi:10.1002/Smll01593.
Xi L, Minati S, Zhao Q, Qian W, Yang L, Jiang H. HER-2/Neu targeted delivery of a nanoprobe enabled dual photoacoustic and fluorescence tomography of ovarian cancer. Nanomedicine. 2013. doi:10.1016/J.Nano.11.004.
Yang L, Mao H, Cao Z, et al. Molecular imaging of pancreatic cancer in an animal tumor model using targeted multifunctional nanoparticles. Gastroenterology. 2009;136(5):1514–25.
Erpelding TN, Kim C, Pramanik M, et al. Sentinel lymph nodes in the rat: noninvasive photoacoustic and US imaging with a clinical US system. Radiology. 2010;256(1):102–10.
Mohs AM, Mancini MC, Singhal S, Provenzale JM, Leyland-Jones B, Wang MD, et al. Hand-held spectroscopic device for in vivo and intraoperative tumor detection: contrast enhancement, detection sensitivity, and tissue penetration. Anal Chem. 2010;82(21):9058–65.
Acknowledgment
We thank Dr. Andrew Y. Wang at Ocean Nanotech, LLC, for providing magnetic IONPs, and Dr. Malgorzata Lipowska at Emory University for synthesis of NIR 830 dye. This research project was supported by the following National Institutes of Health (NIH) Grants: NIH R21CA 161384 (Huabei Jiang) and R01CA133722 (Lily Yang).
Disclosures
No potential conflicts of interest were disclosed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xi, L., Zhou, G., Gao, N. et al. Photoacoustic and Fluorescence Image-Guided Surgery Using a Multifunctional Targeted Nanoprobe. Ann Surg Oncol 21, 1602–1609 (2014). https://doi.org/10.1245/s10434-014-3541-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-3541-9