Abstract
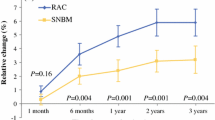
We sought the extent to which arm morbidity could be reduced by using sentinel-lymph-node-based management in women with clinically node-negative early breast cancer. One thousand eighty-eight women were randomly allocated to sentinel-lymph-node biopsy followed by axillary clearance if the sentinel node was positive or not detected (SNBM) or routine axillary clearance (RAC, sentinel-lymph-node biopsy followed immediately by axillary clearance). Sentinel nodes were located using blue dye, alone or with technetium-labeled antimony sulfide colloid. The primary endpoint was increase in arm volume from baseline to the average of measurements at 6 and 12 months. Secondary endpoints were the proportions of women with at least 15% increase in arm volume or early axillary morbidity, and average scores for arm symptoms, dysfunctions, and disabilities assessed at 6 and 12 months by patients with the SNAC Study-Specific Scales and other quality-of-life instruments. Sensitivity, false-negative rates, and negative predictive values for sentinel-lymph-node biopsy were estimated in the RAC group. The average increase in arm volume was 2.8% in the SNBM group and 4.2% in the RAC group (P = 0.002). Patients in the SNBM group gave lower ratings for arm swelling (P < 0.001), symptoms (P < 0.001), and dysfunctions (P = 0.02), but not disabilities (P = 0.5). Sentinel nodes were found in 95% of the SNBM group (29% positive) and 93% of the RAC group (25% positive). SNB had sensitivity 94.5%, false-negative rate 5.5%, and negative predictive value 98%. SNBM was successfully undertaken in a wide range of surgical centers and caused significantly less morbidity than RAC.



Similar content being viewed by others
References
Krag D, Weaver D, Ashikaga T. The sentinel node in breast cancer—a multicentre validation study. N Engl J Med. 1998;339:941–6.
Cody B. Sentinel lymph node biopsy as an alternative to routine axillary lymph node dissection in breast cancer patients. J Surg Oncol. 2001;77(3):149–52.
Cox CE, Salud CJ, Harrington MA. The role of selective sentinel lymph node dissection in breast cancer. Surg Clin North Am. 2000;80(6):1759–77.
Kollias J, Gill PG, Coventry BJ, Malycha P, Chatterton B, Farshid G. Clinical and histological factors associated with sentinel node identification in breast cancer. Aust NZ J Surg. 2000;70:485–9.
Chua B, Ung OA, Taylor R, Bilous M, Salisbury E, Boyages J. Treatment implications of a positive sentinel lymph node biopsy in early breast cancer. Cancer. 2001, 92:1769–74.
Wilkinson D, Wetzig N, Bennett I. Sentinel node biopsy for breast cancer: using local results for estimation of risk to patient. ANZ J Surg. 2003;73:811–4.
Redman S, King E. Encouraging research into lymphoedema: a report on the summit held on 25 and 26 February 2000. Sydney: National Breast and Ovarian Cancer Centre, 2000. http://www.nbcc.org.au/resources/resource.php?code=LRS. Accessed Mar 2008.
Beller EM, Gebski V, Keech AC. Randomisation in clinical trials. Med J Aust. 2002;177:565–7.
ISource National Breast Cancer Centre Early Breast Cancer Working Group. Management of early breast cancer. Canberra: National Health and Medical Research Council; 2001. http://www.nhmrc.gov.au/publications/synopses/_files/cp74.pdf. Accessed April 2008.
Wetzig NR, Gill PG, Ung O, et al. for the RACS SNAC Group. Participation in the RACS Sentinel Node versus Axillary Clearance trial. ANZ J Surg. 2005;75:98–100.
Tewari N, Gill PG, Bochner MA, Kollias J. Comparison of volume displacement versus circumferential arm measurements for lymphoedema—implications for the SNAC trial. ANZ J Surg. 2008;78:889–93.
Nowak AK, Hargreaves C, Gill PG, et al. Health-related quality of life before and 1 month after axillary surgery in a randomized trial of sentinel node biopsy versus axillary clearance: the SNAC trial. In 27th Annual San Antonio Breast Cancer Symposium, 8–11 Dec 2004, San Antonio. Breast Cancer Res Treat. 2004;88 Suppl 1:Abstract 2026.
Smith MJ, Gill PG, Wetzig N, Sourjina T, Gebski V, Ung O, et al. Comparing patients’ and clinicians; assessment of outcomes in a randomised trial of sentinel node biopsy for breast cancer (the RACS SNAC trial). Breast Cancer Res Treat. (in press).
Veronesi U, Paganelli G, Viale G. A randomized comparison of sentinel node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349(6):546–53.
Veronesi U, Paganelli G, Viale G. Sentinel lymph node biopsy as a staging procedure in breast cancer: an update of a randomized controlled study. Lancet Oncol. 2006;7(12):983–90.
Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8(10):881–8.
Mansel RE, Fallowfield LJ, Kissin M. Randomised multicentre trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98(9):599–609.
Fleissig A, Fallowfield LJ, Langridge CI, et al. Post-operative arm morbidity and quality of life. results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer breast. Breast Cancer Res Treat. 2006;95(3):279–93.
Benson JR, Querci della Rovere G, the Axilla Management Consensus Group. Management of the axilla in women with breast cancer. Lancet Oncol. 2007;8:331–48.
Cody HS, Borgen PI. State-of-the-art approaches to sentinel node biopsy for breast cancer: study design, patient selection, technique and quality control at Memorial Sloan-Kettering Cancer Centre. Surg Oncol. 1999;8:85–91.
Adverse Drug Reactions Advisory Committee. Patent blue V and anaphylaxis. Australian Adverse Drug Reactions Bull. 2002;21(3):10. http://www.tga.gov.au/adr/aadrb/aadr0208.htm. Accessed Sep 2008.
Acknowledgements
The study was funded by grants from the Australian National Health and Medical Research Council (NHMRC), the National Breast Cancer Foundation, the Australian Department of Health and Ageing, MBF Australia, and the Scottwood Trust, New Zealand. Educational workshops for investigators were funded by AstraZeneca. Rhana Pike, from the NHMRC Clinical Trials Centre, assisted with the manuscript.
Conflict of Interest
None of the contributors has any conflict of interest.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
A full list of contributors is provided in the Appendix.
Appendix
Appendix
Volume of a Truncated Cone
Consider a segment of length h with a base circumference C b and an apex circumference C a. The area of the base and apex circles is given by A b = C 2b /4Π and A a = C 2a /4Π.
The volume of this segment is
The volume of each arm is then estimated as the sum of the six truncated cones.
Contributors to the SNAC Trial
Writing Committee
P. Grantley Gill (chair), Neil Wetzig, Val Gebski, Martin Stockler, Owen Ung, Ian Campbell, John Simes
Management Committee
P. Grantley Gill (study chair), N. Wetzig (deputy study chair), M. Bilous, I. Campbell, J. Collins, X. Coskinas, G. Farshid, V. Gebski, D. Gillett, W. Hague, R. Harman, J. Kollias, A. Macphee, R.J. Simes, M. Stockler, O. Ung, R. Uren, B. Vachan, L. Young
Safety and Data Monitoring Committee
B. Chatterton, M. Jones, W. Raymond, J. Simpson
Pathology Audit Committee
M. Bilous, G Farshid, X. Coskinas
NHMRC Clinical Trials Centre (Coordinating Centre)
X. Coskinas, A. Ray, K. Scott, B. Vachan (trial coordinators); C. Greig, A. Lucas, R. Tangunan, S. Wonders (data managers); C. Brown, V. Gebski, C. Hargreaves, C. Pardy, T. Sourjina (statisticians); C. Munro, A.-T. Nguyen (database administrators); R. Pike (writer-editor); R.J. Simes (director), M.R. Stockler (oncology co-director)
Sites Contributing Patients to the SNAC Trial and the Principal Investigators
Westmead Hospital, Sydney: Owen Ung, Dominic Moon, James French
Concord Hospital, Sydney: David Gillett, Gail Molland
Coffs Harbour Base Hospital, Coffs Harbour: Bill Ross
Lismore Base Public Hospital, Lismore: Rob Simon
Nepean Hospital, Sydney: Patrick Cregan, Deborah Cheung
Royal Prince Alfred Hospital, Sydney: Andrew Spillane
St Vincent’s Mater Health, Sydney: Margaret Pooley
Strathfield Breast Centre, Sydney: David Gillett, Gail Molland
St. Vincent’s Private Hospital, Lismore: Rob Simon
Baringa Private Hospital, Coffs Harbour: Bill Ross
Royal Melbourne Hospital, Melbourne: John Collins, Bruce Mann, Craig Murphy, Julie Miller
St Vincent’s Hospital, Melbourne: Michael Henderson, Suzanne Moore, Paul Kitchen
Geelong Hospital, Geelong: Gregory Mitchell
Royal Women’s Hospital, Melbourne: Bruce Mann
Princess Alexandra Hospital, Brisbane: Neil Wetzig, David Wilkinson, Ian Bennet
Mater Private Hospital, Brisbane: Neil Wetzig, David Wilkinson, Chris Pyke
Nambour General Hospital, Nambour: Justin D’Arcy, Michael Donovan, Lisa Creighton
Gold Coast Hospital, Southport: Daniel DeVianna
Wesley Medical Centre, Brisbane: Neil Wetzig
Mater Adult Hospital, Brisbane: Chris Pyke
Royal Adelaide Hospital, Adelaide: Grantley Gill, James Kollias, Melissa Bochner, Robert Kennedy
St Andrew’s Hospital, Adelaide: Grantley Gill, James Kollias, Melissa Bochner
Queen Elizabeth Hospital, Adelaide: Vladamir Humeniuk, David Walsh
Western Breast Clinic, Adelaide: Vladamir Humeniuk, David Walsh
Sir Charles Gairdner Hospital, Perth: David Oliver, Diana Hastrich
St John of God Murdoch, Perth: David Oliver
Auckland Hospital, Auckland: Alex Ng
Middlemore Hospital, Auckland: Garth Poole
North Shore Hospital, Auckland: Richard Harman, Sharon Cacala, Eva Juhasz
Palmerston North Hospital, Palmerston North: Colin Wilson, Pravin Kumar, Bruce Rhind
Waikato Hospital, Waikato: Ian Campbell
Principal Site Coordinators
V. Arriola, A. Bell, A. Brown, D. Dash, A. Davis, K. Devantier, A. Dowd, J. Goad, S. Govenlock, J. Hargan, C. Kennedy, K. Latimer, C. MacDonald, C. McBride, L. Neave, M. Osinski, C. Paine, A. Power, C. Preston, J. Scarlet, J. Silbereisen, M. Stanley, P. Whitfield, R. Wicks, R. Winter
Rights and permissions
About this article
Cite this article
Gill, G., The SNAC Trial Group of the Royal Australasian College of Surgeons (RACS) and NHMRC Clinical Trials Centre. Sentinel-Lymph-Node-Based Management or Routine Axillary Clearance? One-Year Outcomes of Sentinel Node Biopsy Versus Axillary Clearance (SNAC): A Randomized Controlled Surgical Trial. Ann Surg Oncol 16, 266–275 (2009). https://doi.org/10.1245/s10434-008-0229-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-008-0229-z