Abstract
Background
It has been suggested that performing a sentinel node biopsy (SNB) in patients with cutaneous melanoma increases the incidence of in-transit metastasis (ITM).
Methods
ITM rates for 2018 patients with primary melanomas ≥1.0 mm thick treated at a single institution between 1991 and 2000 according to 3 protocols were compared: wide local excision (WLE) only (n = 1035), WLE plus SNB (n = 754), and WLE plus elective lymph node dissection (n = 229).
Results
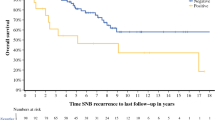
The incidence of ITM for the three protocols was 4.9%, 3.6%, and 5.7%, respectively (not significant), and as a first site of recurrent disease the incidence was 2.5%, 2.4%, and 4.4%, respectively (not significant). The subset of patients who were node positive after SNB and after elective lymph node dissection also had similar ITM rates (10.8% and 7.1%, respectively; P = .11). On multivariate analysis, primary tumor thickness and patient age predicted ITM as a first recurrence, but type of treatment did not. Patients who underwent WLE only and who had a subsequent therapeutic lymph node dissection (n = 149) had an ITM rate of 24.2%, compared with 10.8% in patients with a tumor-positive sentinel node treated with immediate dissection (n = 102; P = .03).
Conclusions
Performing an SNB in patients with melanoma treated by WLE does not increase the incidence of ITM.



Similar content being viewed by others
References
McCarthy JG, Haagensen CD, Herter FP. The role of groin dissection in the management of melanoma of the lower extremity. Ann Surg 1974;179:156–9
Karakousis CP, Choe KJ, Holyoke ED. Biologic behavior and treatment of intransit metastasis of melanoma. Surg Gynecol Obstet 1980;150:29–32
Cascinelli N, Bufalino R, Marolda R, et al. Regional non-nodal metastases of cutaneous melanoma. Eur J Surg Oncol 1986;12:175–80
Singletary SE, Tucker SL, Boddie AW Jr. Multivariate analysis of prognostic factors in regional cutaneous metastases of extremity melanoma. Cancer 1988;61:1437–40
Estourgie SH, Nieweg OE, Valdes Olmos RA, Hoefnagel CA, Kroon BB. Review and evaluation of sentinel node procedures in 250 melanoma patients with a median follow-up of 6 years. Ann Surg Oncol 2003;10:681–8
Calabro A, Singletary SE, Balch CM. Patterns of relapse in 1001 consecutive patients with melanoma nodal metastases. Arch Surg 1989;124:1051–5
Fortner JG, Booher RJ, Pack GT. Results of groin dissection for malignant melanoma in 220 patients. Surgery 1964;55:485–94
Petersen NC, Bodenham DC, Lloyd OC. Malignant melanomas of the skin. A study of the origin, development, aetiology, spread, treatment, and prognosis. Br J Plast Surg 1962;15:97–116
Uren RF, Howman-Giles R, Thompson JF, et al. Interval nodes: the forgotten sentinel nodes in patients with melanoma. Arch Surg 2000;135:1168–72
Uren RF, Howman-Giles R, Thompson JF, et al. Lymphatic drainage to triangular intermuscular space lymph nodes in melanoma on the back. J Nucl Med 1996;37:964–6
Uren RF, Thompson JF, Howman-Giles R, Shaw HM. Melanoma metastases in triangular intermuscular space lymph nodes. Ann Surg Oncol 1999;6:811.
Thompson JF. The Sydney Melanoma Unit experience of sentinel lymphadenectomy for melanoma. Ann Surg Oncol 2001;8:44S–47S
Coates AS, Ingvar CI, Petersen-Schaefer K, et al. Elective lymph node dissection in patients with primary melanoma of the trunk and limbs treated at the Sydney Melanoma Unit from 1960 to 1991. J Am Coll Surg 1995;180:402–9
Morton DL, Thompson JF, Essner R, et al. Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: a multicenter trial. Multicenter Selective Lymphadenectomy Trial Group. Ann Surg 1999;230:453–63; discussion 463–5
Roses DF, Harris MN, Rigel D, Carrey Z, Friedman R, Kopf AW. Local and in-transit metastases following definitive excision for primary cutaneous malignant melanoma. Ann Surg 1983;198:65–9
Wong JH, Cagle LA, Kopald KH, Swisher SG, Morton DL. Natural history and selective management of in transit melanoma. J Surg Oncol 1990;44:146–50
Reintgen DS, Cox C, Slingluff CL Jr, Seigler HF. Recurrent malignant melanoma: the identification of prognostic factors to predict survival. Ann Plast Surg 1992;28:45–9
Lugassy C, Barnhill RL, Christensen L. Melanoma and extravascular migratory metastasis. J Cutan Pathol 2000;27:481.
Barnhill RL. The biology of melanoma micrometastases. Recent Results Cancer Res 2001;158:3–13
Barnhill RL, Lugassy C. Angiotropic malignant melanoma and extravascular migratory metastasis: description of 36 cases with emphasis on a new mechanism of tumour spread. Pathology 2004;36:485–90
Clary BM, Mann B, Brady MS, Lewis JJ, Coit DG. Early recurrence after lymphatic mapping and sentinel node biopsy in patients with primary extremity melanoma: a comparison with elective lymph node dissection. Ann Surg Oncol 2001;8:328–37
Doting MH, Hoekstra HJ, Plukker JT, et al. Is sentinel node biopsy beneficial in melanoma patients? A report on 200 patients with cutaneous melanoma. Eur J Surg Oncol 2002;28:673–8
Gershenwald JE, Berman RS, Porter G, Mansfield PF, Lee JE, Ross MI. Regional nodal basin control is not compromised by previous sentinel lymph node biopsy in patients with melanoma. Ann Surg Oncol 2000;7:226–31
Pawlik TM, Ross MI, Johnson MM, et al. Predictors and natural history of in-transit melanoma after sentinel lymphadenectomy. Ann Surg Oncol 2004;11:S61. (abstract)
Cascinelli N, Belli F, Santinami M, et al. Sentinel lymph node biopsy in cutaneous melanoma: the WHO Melanoma Program experience. Ann Surg Oncol 2000;7:469–74
Chao C, Wong SL, Ross MI, et al. Patterns of early recurrence after sentinel lymph node biopsy for melanoma. Am J Surg 2002;184:520–4
Balch CM, Soong S, Ross MI, et al. Long-term results of a multi-institutional randomized trial comparing prognostic factors and surgical results for intermediate thickness melanomas (1.0 to 4.0 mm). Intergroup Melanoma Surgical Trial. Ann Surg Oncol 2000;7:87–97
Essner R, Conforti A, Kelley MC, et al. Efficacy of lymphatic mapping, sentinel lymphadenectomy, and selective complete lymph node dissection as a therapeutic procedure for early-stage melanoma. Ann Surg Oncol 1999;6:442–9
Thompson JF, McCarthy WH, Bosch CM, et al. Sentinel lymph node status as an indicator of the presence of metastatic melanoma in regional lymph nodes. Melanoma Res 1995;5:255–60
Reintgen D, Albertini J, Berman C, et al. Accurate nodal staging of malignant melanoma. Cancer Control 1995;2:405–14
Robert ME, Wen DR, Cochran AJ. Pathological evaluation of the regional lymph nodes in malignant melanoma. Semin Diagn Pathol 1993;10:102–15
Cochran AJ, Wen DR, Morton DL. Occult tumor cells in the lymph nodes of patients with pathological stage I malignant melanoma. An immunohistological study. Am J Surg Pathol 1988;12:612–8
Wang X, Heller R, VanVoorhis N, et al. Detection of submicroscopic lymph node metastases with polymerase chain reaction in patients with malignant melanoma. Ann Surg 1994;220:768–74
Doubrovsky A, De Wilt JH, Scolyer RA, McCarthy WH, Thompson JF. Sentinel node biopsy provides more accurate staging than elective lymph node dissection in patients with cutaneous melanoma. Ann Surg Oncol 2004;11:829–36
Borgstein PJ, Meijer S, van Diest PJ. Are locoregional cutaneous metastases in melanoma predictable? Ann Surg Oncol 1999;6:315–21
Uren RF, Thompson JF, Howman-Giles R. Lymphatic Drainage of the Skin and Breast: Locating the Sentinel Nodes. Amsterdam: Harwood Academic Publishers, 1999
Uren RF, Howman-Giles RB, Thompson JF, Roberts J, Bernard E. Variability of cutaneous lymphatic flow rates in melanoma patients. Melanoma Res 1998;8:279–82
Curry BJ, Myers K, Hersey P. Utility of tests for circulating melanoma cells in identifying patients who develop recurrent melanoma. Recent Results Cancer Res 2001;158:211–30
Hofer SO, Shrayer D, Reichner JS, Hoekstra HJ, Wanebo HJ. Wound-induced tumor progression: a probable role in recurrence after tumor resection. Arch Surg 1998;133:383–9
Kelly JW, Sagebiel RW, Calderon W, Murillo L, Dakin RL, Blois MS. The frequency of local recurrence and microsatellites as a guide to reexcision margins for cutaneous malignant melanoma. Ann Surg 1984;200:759–63
Heenan PJ, Ghaznawie M. The pathogenesis of local recurrence of melanoma at the primary excision site. Br J Plast Surg 1999;52:209–13
Nicolson GL, Dulski KM. Organ specificity of metastatic tumor colonization is related to organ-selective growth properties of malignant cells. Int J Cancer 1986;38:289–94
Hoon DS, Korn EL, Cochran AJ. Variations in functional immunocompetence of individual tumor-draining lymph nodes in humans. Cancer Res 1987;47:1740–4
Nip J, Shibata H, Loskutoff DJ, Cheresh DA, Brodt P. Human melanoma cells derived from lymphatic metastases use integrin alpha v beta 3 to adhere to lymph node vitronectin. J Clin Invest 1992;90:1406–13
Nakayama T, Taback B, Turner R, Morton DL, Hoon DS. Molecular clonality of in-transit melanoma metastasis. Am J Pathol 2001;158:1371–8
Herbst RA, Mommert S, Casper U, et al. 11q23 allelic loss is associated with regional lymph node metastasis in melanoma. Clin Cancer Res 2000;6:3222–7
Lugassy C, Kleinman HK, Engbring JA, et al. Pericyte-like location of GFP-tagged melanoma cells: ex vivo and in vivo studies of extravascular migratory metastasis. Am J Pathol 2004;164:1191–8
Lugassy C, Haroun RI, Brem H, et al. Pericytic-like angiotropism of glioma and melanoma cells. Am J Dermatopathol 2002;24:473–8
Barnhill R, Dy K, Lugassy C. Angiotropism in cutaneous melanoma: a prognostic factor strongly predicting risk for metastasis. J Invest Dermatol 2002;119:705–6
Acknowledgments
Supported by the Melanoma Foundation of the University of Sydney. D.v.P. was a Sydney Melanoma Unit research student supported by the Dutch Cancer Foundation (Koningin Wilhelmina Fonds), the Dutch Skin Foundation (Stichting Nederlands Huidfonds), and the Dutch Society for Physics and Medical Science (Nederlands Genootschap voor Natuur-, Genees- en Heelkunde). J.G.M. was a Sydney Melanoma Unit research fellow supported by the Walter C. MacKenzie-Scotiabank Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published by Springer Science+Business Media, Inc. © 2005 The Society of Surgical Oncology, Inc.
Rights and permissions
About this article
Cite this article
van Poll, D., Thompson, J.F., Colman, M.H. et al. A Sentinel Node Biopsy Does Not Increase the Incidence of In-Transit Metastasis in Patients With Primary Cutaneous Melanoma. Ann Surg Oncol 12, 597–608 (2005). https://doi.org/10.1245/ASO.2005.08.012
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/ASO.2005.08.012