Abstract
Background: The aim of this study was to assess the capacity of positron emission tomography (PET) with fluorodeoxyglucose (FDG) to determine axillary lymph node status in patients with breast cancer undergoing sentinel node (SN) biopsy.
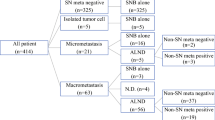
Methods: Thirty-two patients with breast cancer and clinically negative axillary nodes were recruited. All patients underwent FDG-PET before SN biopsy. After SN biopsy, all patients underwent complete axillary lymph node (ALN) dissection.
Results: The SNs were identified in all patients. Fourteen patients (43.8%) had metastatic SNs (macrometastatic in seven, micrometastatic in six, and isolated tumor cells in one). The false-negative rate of SN biopsy was 6.6% (1 in 15). FDG-PET identified lymph node metastases in 3 of the 14 patients with positive SNs. The overall sensitivity, specificity, and positive and negative predictive values of FDG-PET in the diagnosis of axillary metastasis were 20%, 100%, 100%, and 58.6%, respectively. No false-positive findings were obtained with FDG-PET.
Conclusions: This study demonstrates the limitations of FDG-PET in the detection of ALN metastases in patients with early breast cancer. In contrast, FDG-PET seems to be a specific method for staging the axilla in breast cancer. SN biopsy can be avoided in patients with positive FDG-PET, in whom complete ALN dissection should be the primary procedure.
Similar content being viewed by others
REFERENCES
Newcomb PA, Lantz PM. Recent trends in breast cancer incidence, mortality, and mammography. Breast Cancer Res Treat 1993; 28:97–106.
Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin 2001; 51:15–36.
Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 1989; 63:181–7.
Krag DN, Weaver DL, Alex JC, Fairbank JT. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol 1993; 2:335–40.
Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg 1994; 220:391–401.
Nieweg OE, Rutgers EJ, Jansen L, et al. Is lymphatic mapping in breast cancer adequate and safe? World J Surg 2001; 25:780–8.
Greco M, Crippa F, Agresti R, et al. Axillary lymph node staging in breast cancer by 2-fluoro-2-deoxy-d-glucose-positron emission tomography. Clinical evaluation and alternative management. J Natl Cancer Inst 2001; 93:630–5.
Smith IC, Ogston KN, Whitford P, et al. Staging of the axilla in breast cancer: accurate in vivo assessment using positron emission tomography with 2-(fluorine-18)-fluoro-2-deoxy-d-glucose. Ann Surg 1998; 228:220–7.
Avril N, Dose J, Janicke F, et al. Assessment of axillary lymph node involvement in breast cancer patients with positron emission tomography using radiolabeled 2-(fluorine-18)-fluoro-2-deoxy-d-glucose. J Natl Cancer Inst 1996; 88:1204–9.
Utech CI, Young CS, Winter PF. Prospective evaluation of fluorine-18 fluorodeoxyglucose positron emission tomography in breast cancer for staging of the axilla related to surgery and immunocytochemistry. Eur J Nucl Med 1996; 23:1588–93.
Adler LP, Faulhaber PF, Schnur KC, Al-Kasi NL, Shenk RR. Axillary lymph node metastases: screening with [F-18]2-deoxy-2-fluoro-d-glucose (FDG) PET. Radiology 1997; 203:323–7.
Rostom AY, Powe J, Kandil A, et al. Positron emission tomography in breast cancer: a clinicopathological correlation of results. Br J Radiol 1999; 72:1064–8.
Schirrmeister H, Kuhn T, Guhlmann A, et al. Fluorine-18 2-deoxy-2-fluoro-d-glucose PET in the preoperative staging of breast cancer: comparison with the standard staging procedures. Eur J Nucl Med 2001; 28:351–8.
Guller U, Nitzsche EU, Schirp U, et al. Selective axillary surgery in breast cancer patients based on positron emission tomography with 18F-fluoro-2-deoxy-d-glucose: not yet! Breast Cancer Res Treat 2002; 71:171–3.
Yang JH, Nam SJ, Lee TS, Lee HK, Jung SH, Kim BT. Comparison of intraoperative frozen section analysis of sentinel node with preoperative positron emission tomography in the diagnosis of axillary lymph node status in breast cancer patients. Jpn J Clin Oncol 2001; 31:1–6.
Van der Hoeven JJ, Hoekstra OS, Comans EF, et al. Determinants of diagnostic performance of [F-18] fluorodeoxyglucose positron emission tomography for axillary staging in breast cancer. Ann Surg 2002; 236:619–24.
Acland KM, Healy C, Calonje E, et al. Comparison of positron emission tomography scanning and sentinel node biopsy in the detection of micrometastases of primary cutaneous malignant melanoma. J Clin Oncol 2001; 19:2674–8.
Li KY, Shen ZZ. An analysis of 1,242 cases of extended radical mastectomy. Breast 1984; 10:10–9.
Cody HS III, Urban JA. Internal mammary node status: a major prognosticator in axillary node-negative breast cancer. Ann Surg Oncol 1995; 2:32–7.
Cox CE, Bass SS, Reintgen DS. Techniques for lymphatic mapping in breast carcinoma. Surg Oncol Clin North Am 1999; 8:447–67.
Arriagada R, Le MG, Mouriesse H, et al. Long-term effect of internal mammary chain treatment. Results of a multivariate analysis of 1195 patients with operable breast cancer and positive axillary nodes. Radiother Oncol 1988; 11:213–22.
Lacour J, Le MG, Hill C, Kramar A, Contesso G, Sarrazin D. Is it useful to remove internal mammary nodes in operable breast cancer? Eur J Surg Oncol 1987; 13:309–14.
Lacour J, Le M, Caceres E, Koszarowski T, Veronesi U, Hill C. Radical mastectomy versus radical mastectomy plus internal mammary dissection. Ten year results of an international cooperative trial in breast cancer. Cancer 1983; 51:1941–3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barranger, E., Grahek, D., Antoine, M. et al. Evaluation of Fluorodeoxyglucose Positron Emission Tomography in the Detection of Axillary Lymph Node Metastases in Patients With Early-Stage Breast Cancer. Ann Surg Oncol 10, 622–627 (2003). https://doi.org/10.1245/ASO.2003.12.019
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1245/ASO.2003.12.019