-
PDF
- Split View
-
Views
-
Cite
Cite
Claudia De Lorenzo, Rosanna Cozzolino, Andrea Carpentieri, Piero Pucci, Paolo Laccetti, Giuseppe D'Alessio, Biological properties of a human compact anti-ErbB2 antibody, Carcinogenesis, Volume 26, Issue 11, November 2005, Pages 1890–1895, https://doi.org/10.1093/carcin/bgi146
Close - Share Icon Share
Abstract
ErbB2 is a prognostic factor and target of therapy for many carcinomas. In contrast with the other ErbB receptors, ErbB2 lacks a soluble direct ligand, but it is the preferred co-receptor for the ErbB family members, forming heterodimers with more potent and prolonged signalling activity than that of homodimers. We recently produced a new anti-ErbB2 antibody, Erb-hcAb, by fusion of Erbicin, a human, anti-ErbB2 scFv, selectively cytotoxic to ErbB2-positive cells, and a human Fc domain. This fully human antitumour antibody represents a compact version of an IgG1, with the cytotoxicity of the scFv moiety on target cells, combined with the ability of the Fc moiety to induce both antibody- and complement-dependent cytotoxicity. Here, we describe the main properties of Erb-hcAb, using as a reference Herceptin, an anti-ErbB2 humanized monoclonal currently employed in clinical immunotherapy. We found that both bivalent Erb-hcAb and Herceptin increase receptor phosphorylation and downregulation, whereas monovalent Erbicin does not. These results correlate with the finding that Erb-hcAb is capable of inducing apoptosis and inhibiting cell cycle progression in ErbB2-positive cells. Its powerful in vitro antitumour action matched that observed in vivo in experiments with human ErbB2-positive tumour xenografts established in athymic mice. Finally, Erb-hcAb displays a glycosylation profile virtually superimposable to that of a human IgG. These findings suggest that Erb-hcAb is a very promising new agent for the immunotherapy of carcinomas that overexpress the ErbB2 receptor.
Introduction
There is a major interest in considering the overexpression of the ErbB2 receptor as a prognostic factor and target in therapy for breast carcinoma and other carcinomas, such as those of bladder, lung, colon, stomach and pancreas ( 1 – 3 ). In contrast with the other receptors of the ErbB family, ErbB2 lacks a soluble direct ligand, but it is the preferred co-receptor for its family members, namely ErbB-1, ErbB-3 and ErbB-4 ( 4 ). The ErbB receptor heterodimers that contain ErbB2 show higher ligand affinity and lower rate endocytosis than homodimers, hence engender a more potent and prolonged signalling activity ( 5 ). Furthermore, once internalized, heterodimers are targeted for recycling to the cell surface, where they can undergo another cycle of activation; homodimers instead are destined for degradation ( 6 ).
Recent studies propose that anti-ErbB2 antibodies can trigger endocytosis of the receptor through homodimerization ( 7 , 8 ). Once internalized, the ErbB2 homodimers can recruit Cbl ubiquitin ligase and are routed to the degradation pathway ( 9 ). Thus, antibodies can promote the degradation of ErbB2 and remove ErbB2 from the cell surface. A reduced level of ErbB2 at the cell surface induces a decreased formation of ErbB2 heterodimers, resulting in a reduced growth factor-induced signalling and cell proliferation.
We recently developed a new anti-ErbB2 antibody from a human, per se cytotoxic single chain variable fragment (scFv) ( 10 ) and a human Fc domain. This fully human antitumour antibody (named Erbicin-derived human, compact antibody or Erb-hcAb) represents a compact, reduced version of an IgG1, with the antiproliferative effect of the scFv moiety on tumour target cells, combined with the ability of the Fc moiety to induce both antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) ( 11 ). Based on previous studies ( 12 – 14 ), such a compact antibody is expected to have the advantage of the prolonged half-life of an antibody, composed with an increased extravascular diffusion, both very expedient features for targeting solid tumours.
A recombinant, human scFv-Fc antibody has been already reported ( 14 ) to endure a much longer serum half-life in vivo when compared with its parental scFv. However, the protein was produced in yeast with yeast controlled glycosylation; furthermore, it was not characterized, as it was obtained in very low yields, and found to be heterogeneous, i.e. only partially glycosylated. It should be noted that Erb-hcAb was prepared instead in CHO cells, a mammalian model certainly closer than yeast to human cells.
Here, we describe the main properties of Erb-hcAb, a human, compact antibody. Our results suggest that this new antibody is a very promising agent for the immunotherapy of carcinomas that overexpress the ErbB2 receptor.
Materials and methods
Antibodies
The antibodies used in the current study were the following: murine anti-phosphotyrosine monoclonal antibody (mAb) P-Tyr (PY99) (Santa Cruz Biotechnology, Santa Cruz, CA); rabbit polyclonal anti-ErbB2 immunoglobulin antibody (Neu C-18) (Santa Cruz Biotechnology); murine anti-actin mAb (Sigma, St Louis, MO); Herceptin (Genentech, South San Francisco, CA); horseradish peroxidase (HRP) conjugated goat anti-rabbit immunoglobulin antibody (Pierce, Rockford, IL); HRP-conjugated rabbit anti-mouse immunoglobulin antibody (Pierce).
Cell cultures
MDA-MB 361 cells (kindly provided by Dr N.Normanno, Cancer Institute of Naples), SKBR3 cells (from American Type Culture Collection, Rockville, MD), both from human breast carcinomas, and the A431 cell line from human epidermoid carcinoma (from American Type Culture Collection) were cultured in RPMI 1640 (Gibco BRL, Life Technologies, Paisley, UK) supplemented with 10% foetal bovine serum, 50 U/ml penicillin and 50 μg/ml streptomycin (all from Gibco BRL).
Cell lysis and immunoblotting analyses
SKBR3 cell lysates were prepared by resuspending in 0.1 ml lysis buffer (10 mM Tris–HCl, pH 7.4, 150 mM NaCl and 0.5% Nonidet P-40 containing Complete™ proteases inhibitor) (Boehringer Mannheim, Germany) about 1 × 10 6 cells, previously detached with the cell dissociation solution (Sigma), and washed three times in phosphate-buffered saline (PBS). After 20 min, at 0°C the extracts were clarified by centrifugation at 12 000 r.p.m. for 15 min.
Protein concentration was determined by a colorimetric assay (Bradford, Sigma), and aliquots of 20 μg were run on 7.5% SDS–PAGE, followed by electroblotting onto PVDF membranes (Millipore, Bedford, MA). The ErbB2 protein was detected using the anti-ErbB2 sc-284 mAb (Santa Cruz), followed by rabbit anti-mouse horseradish peroxidase-conjugated antibody. The signal intensity of reactive bands was quantitatively measured with a phosphorimager (GS-710; Bio-Rad, Hercules, CA).
Determination of ErbB2 phosphorylation
SKBR3 cells were grown at 37°C in 6-well plates, starved for 24 h in serum-free RPMI medium, then treated with scFv, Erb-hcAb or Herceptin (each at a concentration of 200 nM), in fresh, serum-free medium. After the indicated time periods, cells were washed with PBS, harvested and lysed in the presence of 1 mM sodium orthovanadate (Sigma), as described above. Immunoblotting analyses were performed with an anti-phosphotyrosine mAb. The signal intensity of reactive bands was quantified with a phosphorimager (GS-710; Bio-Rad).
The data were normalized to those obtained for the same extracts using an anti-actin mAb (Sigma).
Downregulation assay
SKBR3 cells were plated at a density of 6 × 10 5 in 6-well plates. After 24 h, the medium was replaced by methionine-free medium containing 100 μCi [ 35 S]methionine per ml and 2% fetal bovine serum. The cells were metabolically labelled for 16 h and then incubated with scFv, Erb-hcAb or Herceptin (each at a concentration of 200 nM) in fresh medium containing unlabelled methionine. After 16 h, whole cell lysates were prepared and equal amounts, determined by c.p.m. measurement, were subjected to immunoprecipitation with the C-terminal-specific anti-ErbB2 antibody Neu C-18 (Santa Cruz) and with anti-actin mAb (Sigma). The immune complexes were collected by adsorption to protein A-Sepharose beads (Sigma) for 1 h at 4°C, then washed and released by boiling in loading buffer. Samples were separated by electrophoresis on a 7.5% SDS–PAGE, and analysed by autoradiography.
The band intensities were quantified by using a scanner (Molecular Imager FX, Biorad).
Cell cycle analysis
SKBR3 cells were seeded in 6-well plates at a density of 6 × 10 5 and incubated at 37°C with Erb-hcAb or Herceptin at a concentration of 100 or 200 nM. After 3 days, cells were detached with the cell dissociation solution (Sigma), washed with PBS and resuspended in PI staining buffer (50 μg/ml propidium iodide, 0.1% NP-40 and 10 μg/ml RNase A). Following 30 min of incubation at room temperature, cell cycle analysis was performed on a FACS Calibur Flow Cytometer (Becton Dickinson, Oxford, UK). The percentage of cells in each phase of the cell cycle (G 1 , S and G 2 /M) was calculated by using Modfit software (Becton Dickinson), and compared with the results obtained with control untreated cells.
Detection of apoptosis
To test apoptotic death, SKBR3 cells were seeded in 6-well plates at a density of 6 × 10 5 /well, in the absence or in the presence of Erb-hcAb or Herceptin (each at a concentration of 200 nM). After 24 or 40 h, cells were harvested, washed in PBS, and treated with Annexin V-FITC following resuspension in binding buffer [10 mM HEPES (pH 7.4), 140 mM NaCl and 2.5 mM CaCl 2 ]. Cells were then stained with propidium iodide according to the manufacturer's recommendations (Becton Dickinson). Labelled cells were analysed using the FACS Calibur flow cytometer (Becton Dickinson); the data were processed using CellQuest software (Becton Dickinson).
The apoptotic inducer puromycin (10 μg/ml) was used as a positive control.
Cytotoxicity assays
Cells were seeded in 96-well plates at a density of 1.5 × 10 4 /well in 150 μl, and Erb-hcAb was added at increasing concentrations. After 72 h, cell viability was determined in triplicate using the ATPlite 1 step test (Perkin Elmer, Cologno Monzese, Italy). The resulting luminescence was measured in a microplate counter (Multilabel Counter Victor 3, Perkin Elmer). Cell survival was expressed as percent of viable treated cells with respect to control untreated cultures. Typically, SD values were <5%.
In vivo antitumour activity
In vivo experiments were performed with 5-week-old female Balb/cByJIco-nu/nu mice (Charles River laboratories, Calco, Italy). MDA-MB 361 cells (5 × 10 5 ) were suspended in 0.2 ml sterile PBS and injected subcutaneously (day 0) in the right paw. At day 11, when tumours were clearly detectable, Erb-hcAb dissolved in PBS was administered intraperitoneally at doses of 1.5 mg/kg of body wt for seven times at 72 h intervals. Equimolar doses of Herceptin (2.25 mg/kg of body wt) were administered intraperitoneally to a second group of animals. Another group of control mice was treated with identical volumes of sterile PBS. During the period of treatment, tumour volumes ( V ) were measured with calliper and calculated by the formula of rotational ellipsoid V = A × B2 /2 ( A is the axial diameter and B the rotational diameter). SD values were <10%. All mice were maintained at the animal facility of the Department of Cellular and Molecular Biology and Pathology, University of Naples Federico II. The animal experimentations described herein were conducted in accordance with the Italian regulation for experimentation on animals. All in vivo experiments were carried out with ethical committee approval and met the standards required by the UKCCCR guidelines ( 15 ).
Chemical and enzymatic hydrolyses
Trypsin, dithiothreitol and iodoacetamide were purchased from Sigma. Peptide N-glycosidase F (PNGase F) was obtained from Roche (Mannheim, Germany). Pre-packed PD-10 gel filtration cartridges were from Pharmacia (Cologno Monzese, Italy). Pre-packed Sep pak C18 cartridges were purchased from Waters (Milano, Italy). All other reagents and solvents were of the highest purity available from Carlo Erba (Rodano, Italy).
ERB-hcAb (500 μg) was reduced and alkylated as previously described ( 16 ) and digested with trypsin in 50 mM ammonium bicarbonate (pH 8.5), overnight at 37°C using 1:50 enzyme/substrate ratio (w/w). Samples were then diluted with water and lyophilized. Deglycosylation of the peptide mixtures was performed by treatment with PNGase F in 50 mM ammonium bicarbonate (pH 8.5), overnight at 37°C using 0.1 U of enzyme per 500 μg of protein. N-linked oligosaccharide chains were separated from peptides by reverse phase chromatography on prepacked Sep-pak cartridges.
Mass spectrometry
Positive MALDI/MS (matrix-assisted laser desorption ionization) analyses and MALDI/PSD (post source decay) experiments were carried out on a Voyager DE-Pro instrument operating in reflectron mode (Applied Biosystems, Framingham, MA). The MALDI matrices were prepared by dissolving 10 mg of 2,5-dihydroxybenzoic acid in 1 ml methanol or 10 mg α-cyano in 1 ml of acetonitrile/0.2% trifluoroacetic acid (70:30 v/v). Typically, 1 μl of matrix was applied to the metallic sample plate and 1 μl of analyte was then added. Acceleration and reflector voltages were set up as follows: target voltage at 20 kV, first grid at 66% of target voltage, delayed extraction at 200 ns. The PSD fragment spectra were acquired after pseudo-molecular cation selection using the time ion selector. Fragment ions were refocused by stepwise reduction of the reflector voltage by 20%.
The individual segments were then stitched together using the Applied Biosystems software. Raw data were analysed using the computer software provided by the manufacturers and are reported as monoisotopic masses.
Results
Structural characterization of Erb-hcAb
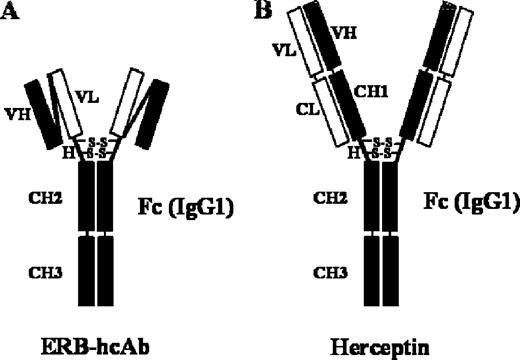
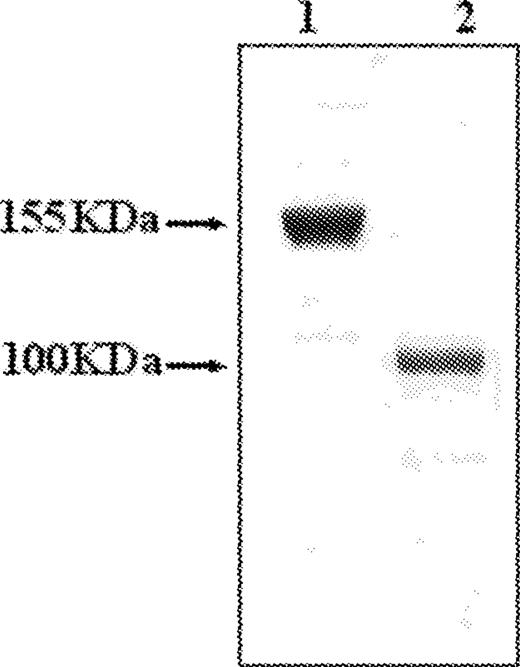
Erb-hcAb, the compact version of a human anti-ErbB2 antibody, i.e. an immunoglobulin-like IgG1 protein with a reduced molecular weight, was prepared as previously described ( 11 ). The resulting construct preserves all functionally relevant antibody regions, even though it lacks the CH1 and CL domains with respect to a full-size immunoglobulin, such as Herceptin ( Figure 1 ). When the compact antibody was analysed by SDS–PAGE ( Figure 2 ), it was found to migrate under non-reducing conditions with a reduced molecular size of ∼100 kDa compared with that of Herceptin (155 kDa).
Schematic representation of Erb-hcAb and Herceptin. ( A ) Erb-hcAb, a human compact anti-ErbB2 antibody; ( B ) Herceptin, a full-size IgG. VH and VL are the heavy and light chain variable domains, respectively. H, the hinge region with disulfide bridges. CH1, CH2, CH3 are the heavy chain constant domains, and CL is the light constant domain of a human IgG1.
Analysis of purified Erb-hcAb by SDS–PAGE. Erb-hcAb (lane 2), and Herceptin (lane 1) were run by SDS–PAGE under non-reducing conditions.
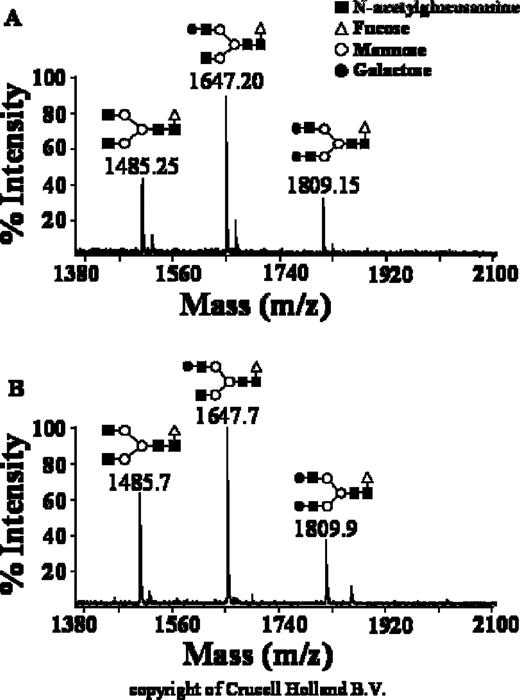
An ideal antibody for immunotherapy should not only contain a reduced convenient molecular size, but also human-type glycan structures, hence increased half-life and biological activity, and low or absent immunogenicity. The structure of the glycosidic moieties of Erb-hcAb was investigated by direct MALDI/MS analysis of the oligosaccharide mixtures in positive ion mode. Figure 3A shows the mass signals recorded in the spectra and the corresponding oligosaccharide structures inferred by MS data. The glycans N-linked to Erb-hcAb belong to the complex type consisting essentially of fucosylated and non-fucosylated biantennary structures.
Glycosylation profile of Erb-hcAb. Mass spectra of glycans from Erb-hcAb ( A ) and a human serum IgG ( B ). Panel B was provided by courtesy of Crucell Holland B.V., Leiden, The Netherlands.
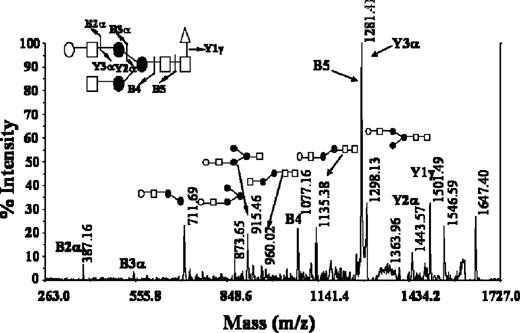
The ultimate definition of the different glycans structures including the sequence and branching degree of the antennae was achieved by tandem mass spectrometry experiments using the MALDI/PSD technique. Individual ions corresponding to the different glycoforms were isolated and fragmented in PSD mode. The characteristic PSD fragmentation pattern, mainly due to ring cleavages and to A-type cleavages occurring at the non-reducing end of N -acetylglucosamine residues, led us to the definition of the structure of glycoforms. As an example, Figure 4 shows the PSD spectrum of Erb-hcAb, obtained by selecting the precursor ion at m / z 1647.2. The mass spectrum exhibited a simple fragmentation pattern in which the complete series of Y ions allowed immediate assignment of the full monosaccharide sequence. The B5 ion at m / z 1279.6 resulting from the cleavage of the glycosidic bond between the two N -acetylhexosamine core residues led to the localization of the fucose moiety on the first HexNAc.
MALDI/PSD spectrum of the m / z 1647.2 ion recorded from Erb-hcAb glycoforms. The Y and B ion series are indicated. Fragmentation events are reported on the glycosidic structure.
As shown in Figure 3 , the glycosylation profile of Erb-hcAb (panel A) is very similar to that observed for human serum IgG (panel B). Levels of galactosylation are equivalent to those seen in human IgG (0-galactose/1-galactose/2-galactose in the ratio 1/2/1). Core fucose is also present and no hybrid structures or high-mannose structures are detectable.
Effects on ErbB2 phosphorylation
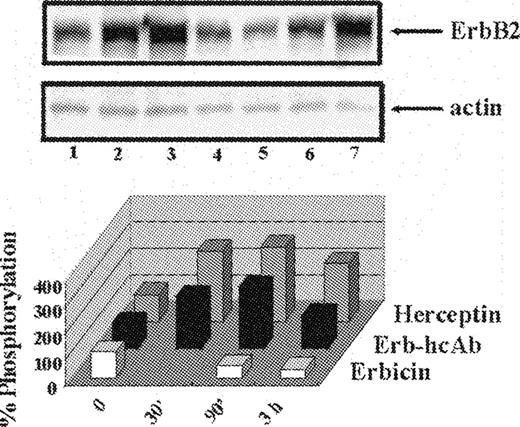
The effects of ERB-hcAb were compared with those of Erbicin, the parental scFv, used as a negative control, and of Herceptin, a humanized anti-ErbB2 mAb currently used for the therapy of breast carcinoma, used as a positive control. Cell extracts of starved SKBR3 cells, treated at 37°C for various time intervals with the immunoagents (200 nM), were analysed by western blottings, using an anti-P-Tyr mAb. The analyses were performed in the presence of an anti-actin mAb to directly compare the levels of ErbB2 receptor phosphorylation. The signal intensity of positive bands was estimated by phosphorimaging. Figure 5 illustrates the results of one of these experiments: Erb-hcAb, as well as Herceptin, effectively stimulate phosphorylation of ErbB2. This is apparently due to their bivalent format and consequent ability to bind two receptors, because monovalent Erbicin does not increase phosphorylation ( Figure 5 ), as it cannot bind simultaneously two receptor molecules and is unable to form homodimers.
The effects of Erb-hcAb on ErbB2 phosphorylation. Upper panel: western blotting analyses with an anti-phosphotyrosine mAb of lysates from SKBR3 cells, untreated (lane 1) or treated for 90 min or 3 h with Erb-hcAb (lanes 2 and 3), Erbicin (lanes 4 and 5) or Herceptin (lanes 6 and 7). Lower panel: the levels of ErbB2 phosporylation are reported as percentages of the phosphorylation level detected in untreated cells.
Effects of Erb-hcAb on ErbB2 levels
One possible mechanism underlying the antiproliferative effects of anti-ErbB2 antibodies is downregulation of the receptor ( 7 , 17 ). We thus analysed whether the effects of the Erbicin-derived compact antibody on tumour cell growth could be attributed to lower ErbB2 levels generated by an increased receptor turnover as triggered by antibody binding.
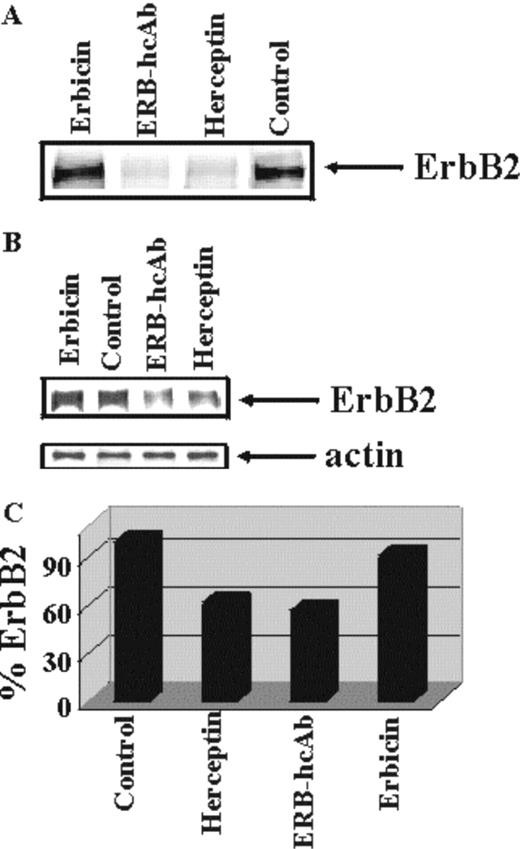
The effects of Erb-hcAb on turnover of ErbB2 was measured on metabolically labelled SKBR3 cells, treated for 16 h with the parental scFv (200 nM), Erb-hcAb (200 nM) or Herceptin (200 nM) used as a control. The receptor was immunoprecipitated with an anti-ErbB2 mAb from aliquots of lysate containing equal amounts of radioactivity, and analysed by SDS–PAGE followed by autoradiography.
Densitometric evaluation of the data showed that the level of ErbB2 was reduced by 40–50% after exposure of SKBR3 cells to Herceptin or Erb-hcAb ( Figure 6 ), whereas no effects were detected upon treatment with the monovalent scFv. This suggests that the bivalent compact antibody has acquired, with respect to the monovalent scFv, the ability to effectively induce the degradation of the receptor.
Turnover of the ErbB2 receptor in SKBR3 cells treated with Erb-hcAb. ( A ) Autoradiograms of immunoprecipitated radioactive ErbB2 from lysates of SKBR3 cells untreated or treated as indicated for 16 h with Erb-hcAb, Erbicin or Herceptin. Equal amounts (c.p.m.) of 35 S-labelled lysates were used. ( B ) Co-immunoprecipitation of ErbB2 and actin, performed for normalization. ( C) The levels of ErbB2 are reported as percentages of the level detected in untreated control cells.
Determination of nature of the tumour cell death
To determine whether the mechanism of cell death occurs through induction of apoptosis, we used annexin V to measure the appearance of phosphatidylserine, a marker of apoptosis, on the outer leaflet of the plasma membrane of SKBR3 cells. Cells treated with Erb-hcAb, as well as those treated with Herceptin, were found to bind more FITC-conjugated annexin V than untreated cells ( Table I ). These results show that the nature of cell death as induced by Erb-hcAb is apoptotic.
Extent of apoptosis in SKBR3 cells treated for 24 or 40 h with Erb-hcAb or Herceptin
| . | Apoptotic cells (%) . | . | MFI a ratio (treated/control cells) . | |
|---|---|---|---|---|
. | 24 h . | 40 h . | . | |
| Control cells | 3 | 5 | 1 | |
| Erb-hcAb | 19 | 31 | 2 | |
| Herceptin | 12 | 32 | 2 | |
| Puromycin b | 41 | 2.3 | ||
| . | Apoptotic cells (%) . | . | MFI a ratio (treated/control cells) . | |
|---|---|---|---|---|
. | 24 h . | 40 h . | . | |
| Control cells | 3 | 5 | 1 | |
| Erb-hcAb | 19 | 31 | 2 | |
| Herceptin | 12 | 32 | 2 | |
| Puromycin b | 41 | 2.3 | ||
MFI is the mean fluorescence intensity measured by flow cytometry analyses after treatment of SKBR3 cells with FITC-conjugated Annexin V.
Puromycin (10 μg/ml) was used as a positive control.
Extent of apoptosis in SKBR3 cells treated for 24 or 40 h with Erb-hcAb or Herceptin
| . | Apoptotic cells (%) . | . | MFI a ratio (treated/control cells) . | |
|---|---|---|---|---|
. | 24 h . | 40 h . | . | |
| Control cells | 3 | 5 | 1 | |
| Erb-hcAb | 19 | 31 | 2 | |
| Herceptin | 12 | 32 | 2 | |
| Puromycin b | 41 | 2.3 | ||
| . | Apoptotic cells (%) . | . | MFI a ratio (treated/control cells) . | |
|---|---|---|---|---|
. | 24 h . | 40 h . | . | |
| Control cells | 3 | 5 | 1 | |
| Erb-hcAb | 19 | 31 | 2 | |
| Herceptin | 12 | 32 | 2 | |
| Puromycin b | 41 | 2.3 | ||
MFI is the mean fluorescence intensity measured by flow cytometry analyses after treatment of SKBR3 cells with FITC-conjugated Annexin V.
Puromycin (10 μg/ml) was used as a positive control.
Effects of Erb-hcAb on cell cycling of ErbB2-overexpressing cells
To test whether a growth arrest contributes to the antitumour action of Erb-hcAb, SKBR3 cells were treated with Erb-hcAb, or Herceptin, for 72 h at 37°C, and then subjected to cell cycle analysis by flow cytometry.
As shown in Table II , Erb-hcAb significantly reduces the percentage of SKBR3 cells undergoing S-phase and produces an accumulation of cells in G 0 /G 1 phase. The effect of Erb-hcAb on the cell cycle is dose-dependent, with a stronger inhibitory effect occurring at higher antibody concentrations ( Table II ). Similar results were obtained with Herceptin ( Table II ), used as a positive control.
Cell cycle analyses of SKBR3 cells treated with Erb-hcAb or Herceptin
. | G 0 /G 1 (% cells) . | S (% cells) . | G 2 /M (% cells) . |
|---|---|---|---|
| Control | 78.7 | 18.2 | 3.04 |
| Erb-hcAb (200 nM) | 87.15 | 10.6 | 2.16 |
| Erb-hcAb (400 nM) | 90.52 | 7.6 | 1.87 |
| Herceptin (200 nM) | 87.04 | 9.49 | 3.47 |
| Herceptin (400 nM) | 89.26 | 7.95 | 2.79 |
. | G 0 /G 1 (% cells) . | S (% cells) . | G 2 /M (% cells) . |
|---|---|---|---|
| Control | 78.7 | 18.2 | 3.04 |
| Erb-hcAb (200 nM) | 87.15 | 10.6 | 2.16 |
| Erb-hcAb (400 nM) | 90.52 | 7.6 | 1.87 |
| Herceptin (200 nM) | 87.04 | 9.49 | 3.47 |
| Herceptin (400 nM) | 89.26 | 7.95 | 2.79 |
Cell cycle analyses of SKBR3 cells treated with Erb-hcAb or Herceptin
. | G 0 /G 1 (% cells) . | S (% cells) . | G 2 /M (% cells) . |
|---|---|---|---|
| Control | 78.7 | 18.2 | 3.04 |
| Erb-hcAb (200 nM) | 87.15 | 10.6 | 2.16 |
| Erb-hcAb (400 nM) | 90.52 | 7.6 | 1.87 |
| Herceptin (200 nM) | 87.04 | 9.49 | 3.47 |
| Herceptin (400 nM) | 89.26 | 7.95 | 2.79 |
. | G 0 /G 1 (% cells) . | S (% cells) . | G 2 /M (% cells) . |
|---|---|---|---|
| Control | 78.7 | 18.2 | 3.04 |
| Erb-hcAb (200 nM) | 87.15 | 10.6 | 2.16 |
| Erb-hcAb (400 nM) | 90.52 | 7.6 | 1.87 |
| Herceptin (200 nM) | 87.04 | 9.49 | 3.47 |
| Herceptin (400 nM) | 89.26 | 7.95 | 2.79 |
In vivo antitumour activity of Erb-hcAb
SKBR3 cells, used for in vitro characterization of Erb-hcAb, are not tumorigenic in athymic mice ( 18 ), hence for in vivo studies Erb-hcAb was tested on the ErbB2-positive MDA-MB 361 tumour cells from human mammary carcinoma ( 19 ). The level of ErbB2 expression in the latter cells was found to be comparable to that measured in SKBR3 cells when a quantitative analysis by western blotting was performed on lysates from the two cell types. In line with this finding, the antitumour effect of Erb-hcAb on the latter cell line (data not shown) was found to be similar to that previously reported for SKBR3 cells ( 11 ).
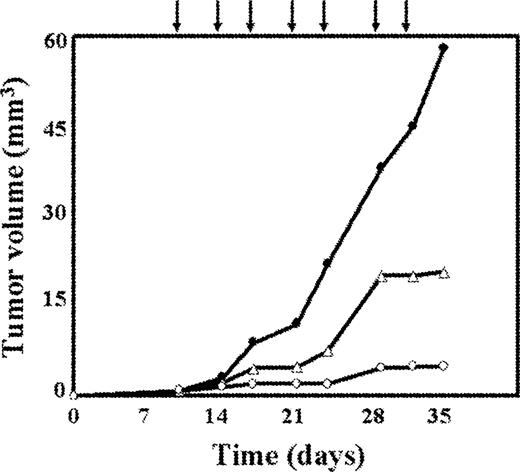
As shown in Figure 7 , the treatment of mice bearing MDA-MB 361 tumours with 7 doses, at 72 h intervals, of 1.5 mg/kg of body wt of Erb-hcAb induced a dramatic reduction in tumour volume. No growth inhibition was observed in untreated mice (data not shown), or in mice treated with PBS ( Figure 7 ). We tested in a parallel experiment the effects of equimolar doses of Herceptin (2.25 mg/kg of body wt) on the same experimental model. The antitumour effect of Herceptin, when compared with that of Erb-hcAb, was found to be less effective ( Figure 7 ). During the period of treatment the animals did not show signs of wasting or other visible signs of toxicity.
In vivo inhibition of tumour growth by Erb-hcAb. Tumour growth was measured in mice inoculated subcutaneously with MDA-MB 361 cells from human mammary carcinoma. Treated mice (open circles) were injected with Erb-hcAb at doses of 1.5 mg/kg of body weight, starting at day 11. Injections were repeated seven times at 72 h intervals as indicated by the arrows. Control mice were treated with sterile PBS solution (black circles), or equimolar doses of Herceptin (open triangles).
Discussion
The understanding of the molecular basis of the mechanism of action of an immunoagent is essential for its exploitation as an antitumour agent. In the present study, we have examined the biological effects of Erb-hcAb on ErbB2-positive cells in comparison with a well-characterized, humanized anti-ErbB2 antibody, such as Herceptin, currently used for treatment of advanced breast cancer ( 20 , 21 ).
Previously, an antineoplastic effect of Erb-hcAb stronger than that of Herceptin was observed ( 11 ) on tumours similar to the alveolar-type human lobular mammary carcinomas, as induced in mice inoculated with murine TUBO cells ( 22 ). In the experiments reported here, we tested for the first time Erb-hcAb on human ErbB2-positive tumour xenografts established in athymic mice. To directly compare the antitumour efficacy of this novel immunoagent with that of Herceptin, we tested in parallel experiments, the effects of equimolar doses of Herceptin on the same experimental model. We found that Erb-hcAb completely inhibited the growth of tumours during the course of treatment, and its effect was significantly more potent than that of Herceptin.
This finding deserves to be commented upon, especially because the affinity of Erb-hcAb for ErbB2 (Kd = 1 nM) is of the same order as that of Herceptin [Kd = 5 nM, see ref. ( 11 )]. However, Erb-hcAb is of a smaller size than Herceptin (∼100 versus 155 kDa), a property which has been shown to promote an increased extra-vascular diffusion and tumour penetration ( 12 , 13 ). It may not be excluded that the ability of Erb-hcAb to induce CDC, a characteristic which is not shared by Herceptin ( 11 , 23 ), might play a role in the higher effectiveness of Erb-hcAb as an antitumour agent in vivo .
We found on the other hand that both antibodies, Erb-hcAb and Herceptin, induce a similar increase in the phosphotyrosine content of ErbB2. The ability of antibodies to affect the extent of ErbB2 phosphorylation reflects their capacity to induce homodimerization of ErbB2 ( 17 ). This presumably leads to its downregulation and degradation. In this respect, we observed a strong reduction of the cellular level of ErbB2 in cells treated with Erb-hcAb.
It is likely that downmodulation of the receptor results from the ability of bivalent Erb-hcAb to cross-link cell surface receptors, since Erbicin, as a monovalent scFv, does not increase ErbB2 phosphorylation, or its degradation.
The downstream effects of antibody-induced down-regulation of ErbB2 could translate to signals that inhibit progression through the cell cycle and enhance apoptosis. Consistent with this hypothesis is the finding reported here that Erb-hcAb is capable of inducing apoptosis and inhibiting cell cycle progression in ErbB2-positive cells.
According to previous studies ( 24 , 25 ), another property of interest to define the potential of Erb-hcAb as an anti-carcinoma immunoagent is the glycosylation of the antibody. We found that the glycosylation profile of Erb-hcAb is virtually superimposable on that of a human IgG. This finding can strengthen the suggestion of a low degree, or absence, of host-defence reactivity in human patients toward Erb-hcAb, an immunoagent of human origin.
These promising findings suggest that Erb-hcAb could be a new, potentially effective and safe antitumour drug.
The authors wish to thank Dr G.Terrazzano for the analyses of apoptotic cells by FACS. This work was supported by AIRC (Associazione Italiana per la Ricerca sul Cancro), Italy.
Conflict of Interest Statement : None declared.
References
Ishida,T., Tsujisaki,M., Hanzawa,Y., Hirakawa,T., Hinoda,Y., Imai,K. and Yachi,A. (
Slamon,D.J., Godolphin,W., Jones,L. et al . (
Lohrisch,C. and Piccart,M. (
Graus-Porta,D., Beerli,R.R., Daly,J.M. and Hynes,N.E. (
Klapper,L.N., Kirschbaum,M.H., Sela,M. and Yarden,Y. (
Yarden,Y. (
Hurwitz,E., Stancovski,I., Sela,M. and Yarden,Y. (
Frankel,A.E. (
Klapper,L.N., Waterman,H., Sela,M. and Yarden,Y. (
De Lorenzo,C., Palmer,D.B., Piccoli,R., Ritter,M.A. and D'Alessio,G. (
De Lorenzo,C., Tedesco,A., Terrazzano,G., Cozzolino,R., Laccetti,P., Piccoli,R. and D'Alessio,G. (
Demignot,S., Pimm,M.V. and Baldwin,R.W. (
Yokota,T., Milenic,D.E., Whitlow,M., Wood,J.F., Hubert,S.L. and Schlom,J. (
Powers,D.B., Amersdorfer,P., Poul,M., Nielsen,U.B., Shalaby,M.R., Adams,G.P., Weiner,L.M. and Marks,J.D. (
Workman,P., Twentyman,P., Balkwill,F. et al . (
Fini,C., Amoresano,A., Andolfo,A., D'auria,S., Floridi,A., Paolini,S. and Pucci,P. (
Klapper,L.N., Vaisman,N., Hurwitz,E., Pinkas-Kramarski,R., Yarden,Y. and Sela,M. (
Pegram,M., Hsu,S., Lewis,G., Pietras,R., Beryt,M., Sliwkowski,M., Coombs,D., Baly,D., Kabbinavar,F. and Slamon,D. (
Ueno,N.T., Bartholomeusz,C., Xia,W. et al . (
Stebbing,J., Copson,E. and O'Reilly,S. (
Baselga,J., Tripathy,D., Mendelsohn,J. et al . (
Rovero,S., Amici,A., Carlo,E.D. et al . (
Drebrin,J.A., Link,V.C. and Greene,M.I. (
Mimura,Y., Church,S., Ghirlando,R., Ashton,P.R., Dong,S., Goodall,M., Lund,J. and Jefferis,R. (
Author notes
Department of Structural and Functional Biology, University of Naples ‘Federico II’, Via Mezzocannone 16, 80134 Naples, Italy and 1Department of Organic Chemistry and Biochemistry, University of Naples, ‘Federico II’, Via Cinthia, 80126 Naples, Italy