Abstract
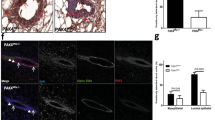
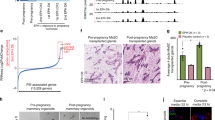
Using a Cre-lox-based genetic labeling technique, we have recently discovered a parity-induced mammary epithelial subtype that is abundant in nonlactating and nonpregnant, parous females. These mammary epithelial cells serve as alveolar progenitors in subsequent pregnancies, and transplantation studies revealed that they possess features of multipotent progenitors such as self-renewal and the capability to contribute to ductal and alveolar morphogenesis. Here, we report that these cells are the cellular targets for transformation in MMTV-neu transgenic mice that exhibit accelerated mammary tumorigenesis in multiparous animals. The selective ablation of this epithelial subtype reduces the onset of tumorigenesis in multiparous MMTV-neu transgenics. There is, however, experimental evidence to suggest that parity-induced mammary epithelial cells may not be the only cellular targets in other MMTV-promoter-based transgenic strains. In particular, the heterogeneous MMTV-wnt1 lesions predominantly express the ductal differentiation marker Nkcc1 that is absent in MMTV-neu-derived tumors. Our observations support the idea that tumors originate from distinctly different epithelial subtypes in selected MMTV-promoter-driven cancer models and that diverse oncogenes might exert discrete effects on particular mammary epithelial subtypes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, McMahon JA, McMahon AP and Weinberg RA . (2000). Genes Dev., 14, 650–654.
Cardiff RD, Anver MR, Gusterson BA, Hennighausen L, Jensen RA, Merino MJ, Rehm S, Russo J, Tavassoli FA, Wakefield LM, Ward JM and Green JE . (2000). Oncogene, 19, 968–988.
Carstens MJ, Krempler A, Triplett AA, van Lohuizen M and Wagner K-U . (2004) (submitted).
Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD and Muller WJ . (1992). Proc. Natl. Acad. Sci. USA, 89, 10578–10582.
Kisseberth WC and Sandgren EP . (2004). Cancer Res., 64, 857–863.
Kordon EC, McKnight RA, Jhappan C, Hennighausen L, Merlino G and Smith GH . (1995). Dev. Biol., 168, 47–61.
Krempler A, Henry MD, Triplett AA and Wagner KU . (2002). J. Biol. Chem., 277, 43216–43223.
Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, Tan LK, Rosen JM and Varmus HE . (2003). Proc. Natl. Acad. Sci. USA, 100, 15853–15858.
Liu BY, McDermott SP, Khwaja SS and Alexander CM . (2004). Proc. Natl. Acad. Sci. USA, 101, 4158–4163.
Medina D and Smith GH . (1999). J. Natl. Cancer Inst., 91, 967–969.
Reed W, Sandstad B, Holm R and Nesland JM . (2003). Int. J. Surg. Pathol., 11, 65–74.
Robinson GW, McKnight RA, Smith GH and Hennighausen L . (1995). Development, 121, 2079–2090.
Rowse GJ, Ritland SR and Gendler SJ . (1998). Cancer Res., 58, 2675–2679.
Russo IH and Russo J . (1996). Environ. Health Perspect., 104, 938–967.
Shillingford JM, Miyoshi K, Flagella M, Shull GE and Hennighausen L . (2002). Mol. Endocrinol., 16, 1309–1321.
Soriano P . (1999). Nat. Genet., 21, 70–71.
Wagner KU, Boulanger CA, Henry MD, Sgagias M, Hennighausen L and Smith GH . (2002). Development, 129, 1377–1386.
Wagner KU, Krempler A, Qi Y, Park K, Henry MD, Triplett AA, Riedlinger G, Rucker III EB and Hennighausen L . (2003). Mol. Cell. Biol., 23, 150–162.
Wagner KU, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L, Li M, Furth PA and Hennighausen L . (1997). Nucleic Acids Res., 25, 4323–4330.
Wellings SR, Jensen HM and Marcum RG . (1975). J. Natl. Cancer Inst., 55, 231–273.
Acknowledgements
This work was supported, in part, by the Susan G Komen Breast Cancer Foundation (BCTR0402956) and a Public Health Service grant CA93797 from the National Cancer Institute. Support provided to KUW by the Nebraska Cancer and Smoking Disease Research Program (NE DHHS LB506) was imperative to finance in part the maintenance and analysis of the animal models. The UNMC Histology Core Facility is supported by the NCI Cancer Center Grant CA036727. We are grateful to Dr Robert D Cardiff (UC Davis) for his insight into the comparative analysis of histopathological features of mammary neoplasia and for providing histological slides from MMTV-wnt1 tumors. We also thank Jim Turner (National Institutes of Health, Bethesda) for contributing the Nkcc1 antibody to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Henry, M., Triplett, A., Oh, K. et al. Parity-induced mammary epithelial cells facilitate tumorigenesis in MMTV-neu transgenic mice. Oncogene 23, 6980–6985 (2004). https://doi.org/10.1038/sj.onc.1207827
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1207827
Keywords
This article is cited by
-
Fatty acid amide hydrolase drives adult mammary gland development by promoting luminal cell differentiation
Cell Death Discovery (2024)
-
Dual recombinase action in the normal and neoplastic mammary gland epithelium
Scientific Reports (2021)
-
p63 suppresses the ability of pregnancy-identified mammary epithelial cells (PIMECs) to drive HER2-positive breast cancer
Cell Death & Disease (2021)
-
Efficient tissue-type specific expression of target genes in a tetracycline-controlled manner from the ubiquitously active Eef1a1 locus
Scientific Reports (2020)
-
Redirecting Normal and Cancer Stem Cells to a Mammary Epithelial Cell Fate
Journal of Mammary Gland Biology and Neoplasia (2019)