Abstract
The clinical applications of 18F-fluoro-2-deoxyglucose (18FDG) positron emission tomography (PET) in oncology are becoming established. While simple static scanning techniques are used for the majority of routine clinical examinations, increasing use of PET in clinical trials to monitor treatment response with 18FDG and novel tracers reflecting different pharmacodynamic end points, often necessitates a more complex and quantitative analysis of radiopharmaceutical kinetics. A wide range of PET analysis techniques exist, ranging from simple visual analysis and semiquantitative methods to full dynamic studies with kinetic analysis. These methods are discussed, focusing particularly on the available methodologies that can be utilised in clinical trials.
Similar content being viewed by others
Main
18F-fluoro-2-deoxyglucose (18FDG) positron emission tomography (PET) is an important tool in oncology, at the forefront of functional and molecular imaging techniques. Its use has progressed from staging purposes to the assessment of response to treatment (Figure 1). The information derived from 18FDG PET scans is more frequently becoming the basis for decisions on subsequent management, such as the cessation or switching of chemotherapy regimes and the addition or modification of radiotherapy. It is also used clinically to establish the diagnosis of relapse following treatment.
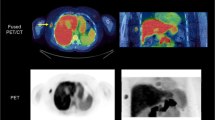
A liver metastasis from a gastric GIST before (A) and 1 week after (B) commencing imatinib therapy. FDG PET/CT scans, unenhanced CT (left), fused FDG PET and CT (right). Although there has been no morphological change in the metastasis, the abnormal baseline metabolic activity (colour scale) has rapidly resolved indicating sensitivity to the drug. The SUV fell from 5.0 to 1.8.
There is a wealth of literature reporting on the role of 18FDG PET in malignancy, showing a wide range of associated sensitivities and specificities; frequently showing greater accuracy than other forms of conventional imaging in a number of tumour types. One of the explanations for the range of reported results may be due to the variability of scanning protocols and analytic techniques used. The analytic techniques range from simple visual qualitative assessment and semiquantitative methods, for example, standardised uptake value (SUV) measurement, to full kinetic analysis with compartmental modelling, for example, nonlinear regression analysis (NLR). A variety of analytical methods have been proposed that need to be validated for different tumour types and for assessing therapeutic response. While simple qualitative techniques are sufficient for many routine clinical examinations, an increase in the use of PET with 18FDG, and also novel tracers reflecting different pharmacodynamic end points in clinical trials, often necessitates a more complex and quantitative approach.
Physiology of 18FDG uptake
Accelerated glucose metabolism is the basis for the use of 18FDG PET in detecting cancer. 18F-fluoro-2-deoxyglucose is transported into glucose-consuming cells with the aid of transporters such as GLUT-1, where it is phosphorylated by hexokinases. Overexpression of glucose transporters and hexokinases has been reported in many cancer cells, leading to their increased accumulation of phosphorylated 18FDG (Smith, 2001). Unless dephosphorylation occurs by glucose-6-phosphatase, 18FDG-6-PO4 remains essentially trapped in the cell. After initial accumulation, there is often rapid dephosphorylation and clearing of FDG-6-PO4 in tissues and organs with high glucose-6-phosphatase activity (Caraco et al, 2000). Malignant cells mostly show low levels of glucose-6-phosphatase compared with many normal tissues and benign pathological processes such as inflammation, leading to different kinetics of 18FDG accumulation between benign and malignant processes (Yamada et al, 1995; Lodge et al, 1999; Nakamoto et al, 2000).
Factors affecting 18F-FDG uptake and scan interpretation
Pathological processes other than malignancy can be responsible for some degree of initial increase in uptake, predominantly infective, inflammatory and granulomatous disorders (e.g., tuberculosis, aspergillosis, sarcoidosis, amyloidosis and postradiotherapy reactions) (Cook et al, 1996). This is thought to be due to uptake in the increased number of activated white cells involved in these processes and can produce a false-positive scan (Kaim et al, 2002). Uptake also depends on various other factors, such as body habitus, insulin levels and blood glucose (Huang, 2000). 18F-fluoro-2-deoxyglucose accumulation is influenced by tumour blood flow but other factors such as glucose transport and hexokinase activity are frequently rate-limiting steps, although a single factor probably does not account for 18FDG uptake in all tumours (Smith, 2001).
The performance characteristics of the PET scanner, scan timing and duration, measurement of injected activity and a number of other technical factors can all affect precision and accuracy of quantitative measurements. The spatial resolution of a PET scanner is usually between 5 and 8 mm in the reconstructed image. Uptake in structures less than two to three times this size can be underestimated as a result of the partial volume effect. For example, a sphere with a diameter equivalent to 1.5 times the spatial resolution will have a maximum measured activity concentration of about 60% of the true activity concentration and a mean of about 30% (Geworski et al, 2000). Heterogeneity of uptake in tumours (e.g., when areas of viable and necrotic tumour exist) can cause similar problems in accurate quantitation of viable tumour activity. Higher activity in surrounding tissues to the region of interest (ROI) can contribute to activity within the ROI by the spillover effect and this may also affect noninvasive measurements of arterial activity concentration (e.g., aorta or left ventricle) when used as a noninvasive method of obtaining an arterial input function (IF) for kinetic analytical methods (Cook et al, 1999).
The recent introduction of hybrid PET/CT (computed tomography) scanners allows combined modality acquisitions in a single sitting. The anatomical detail provided by CT allows better localisation of sites of physiologic activity and pathology identified by PET, thereby improving image interpretation and also providing rapid, low-noise transmission CT data for attenuation correction of PET emission data, essential for quantitative measurements.
A range of software platforms and packages are available for PET data processing and analysis offering different levels of flexibility and complexity to the user (Ratib, 2004). The increasing use of PET/CT has also led to the demand for better software platforms for the reconstruction and optimal display and analysis of both sets of images in a user-friendly format.
Time course of 18FDG uptake and the optimum time of image acquisition
Studies have investigated uptake over time in malignant cells, some tumour-specific, for example, lung cancer and sarcomas, comparing uptake with that in benign and inflammatory lesions (Lodge et al, 1999; Matthies et al, 2002). The maximum uptake in benign tissues occurs earlier at approximately 30 min, with peak uptake in inflammatory tissues occurring at about 60 min (Yamada et al, 1995). There is a wider spectrum for malignant tissues, with peak uptake occurring later, sometimes up to 4 h (Lodge et al, 1999). More consistent and reliable measurements are likely to be possible when tumour tracer concentration has reached a plateau, as small differences in the timing of scans after injection are less likely to affect measured parameters of uptake. This creates difficulties in establishing an optimum scanning time as the half-life of the radioisotope must also be considered (110 min for 18F). The EORTC and NCI guidelines both recommend an optimum scanning time of 50–70 min (Young et al, 1999; Shankar et al, 2006), and this is usually the time taken for clinical scans as a best compromise between tumour to background contrast and decay of tracer.
Methods of image acquisition
Static emission scans are made during a period when the activity distribution is assumed to be fairly stable with a counting time long enough to obtain a good quality image, usually between 3 and 5 min on modern scanners with three-dimensional acquisition. Several bed positions (each covering between 10 and 20 cm) are required to image the whole body. Dynamic scans are carried out by acquiring a continuous series of image frames that can be of various lengths. Early frames are usually shorter to allow kinetic measurements when tracer concentrations are changing rapidly, with longer frames towards the end of a study. Dynamic emission scans are usually acquired from 0 to 60 min for 18FDG. One bed position only is used, limiting the area visualised. Region of interests can be placed and a tissue time–activity curve calculated. Some form of vascular IF is required for analysis for some of the more complex kinetic analytical methods. This can be derived from direct arterial sampling or indirectly by measuring activity over an arterial ROI.
Image analysis
Qualitative analysis
Qualitative interpretation involves simple visual analysis of a static scan by the reporter. Interpretation is based on contrast in uptake between a lesion and surrounding tissues and is dependent on uptake time, blood glucose and insulin levels, and also the nature of the surrounding tissues. In small tumours with low uptake close to normal tissue with high uptake, lesions may become inconspicuous. Following therapy, normal tissue may also undergo physiological changes. For these reasons, visual subjective analysis has poor reproducibility and although it may be suitable for some routine clinical work, it is often insufficient for clinical trials where objective parameters of response are usually required and recommended (Weber, 2005; Shankar et al, 2006).
Semiquantitative analysis
Tumour-to-background normal tissue ratio (T/N) requires a static image and is potentially subject to some of the weaknesses of visual analysis. Even when there has been no change in uptake in malignant tissues, a change in the normal tissue uptake post therapy can lead to a change in the T/N ratio. It is generally regarded as a less reliable index than the SUV. The SUV is the ratio between the tumour concentration of 18FDG and its concentration in the entire body if the tracer is uniformly distributed throughout. It has also been referred to as the differential uptake ratio or the differential absorption ratio. Its measurement requires a static, attenuation-corrected scan, accurate calibration and body weight for normalisation. Normalisation to body surface area or lean body mass may be advantageous. There is also debate about whether it should be corrected for blood glucose, as an improvement in precision has been suggested, but there is no consensus (Thie, 2004). Standardised uptake values are dependent on the uptake time but are most commonly measured at 60 min after injection. Cutoff values for differentiating between benign and malignant tissue vary among different malignancies, often lying between 2 and 3, but this may be variable for different types of tumour. Best documented is the cutoff of 2.5 used in pulmonary lesions (Lowe et al, 1998). There is frequently an area of overlap that can limit its use in an individual patient, some tumours not being 18FDG-avid and some benign processes showing high 18FDG uptake. It is a more reliable index to monitor change within the same patient and is reasonably precise, with less than 10% variation reported in pretreatment scans providing attention is paid in performing consecutive scans in exactly the same way (Minn et al, 1995).
Standardised uptake values are computationally simple and make some of the same assumptions as qualitative analysis. The measured 18FDG concentration is the sum of phosphorylated intracellular 18FDG, nonphosphorylated intracellular 18FDG and nonphosphorylated extracellular (intravascular and interstitial) 18FDG. Of these components, the first is most directly related to the metabolic activity of tumour cells. Static scans cannot differentiate between the different components and therefore do not necessarily correlate with glucose metabolic rates. They do not take into account the physiological changes affecting the availability of 18FDG. This may be of particular importance in assessing response to therapy, when there may be changes in the distribution of 18FDG throughout the body and also in the uptake by the lesion.
Dual-point scanning often uses semiquantitative indices such as SUV but exploits the different kinetics between benign and malignant tissue by comparing activity at two time points, usually at 60 and 120 min, with a number of studies reporting better tissue characterisation when benign and malignant lesions exist (Yamada et al, 1995; Nakamoto et al, 2000; Matthies et al, 2002; Ratib, 2004).
Quantitative analysis
Simplified kinetic analysis
Simplified kinetic analysis is a generic term for methods that attempt to estimate tumour glucose metabolic rate without the need for full dynamic studies or arterial blood measurements but at the same time overcoming some of the limitations of SUVs. They usually rely on a single time point (SKA-S) static image and a single timed blood sample (Hunter et al, 1996), but, more recently, multiple time point (SKA-M) methods have been proposed (Sundaram et al, 2004) with reports of lower variability and bias compared with SKA-S.
Kinetic analysis
Full quantitative analysis uses kinetic modelling approaches to derive the metabolic rate for glucose (MRglu) or 18FDG (MRFDG). Dynamic studies follow metabolic activity over a longer period of time, using a more complex model of the underlying physiology and seeking to remove more of the effects of confounding factors. They are less dependent on the time of measurement. The kinetic models are based on the concept of several compartments that contain 18FDG, linked by kinetic processes that provide a mechanism of exchange of 18FDG (Willemsen and Van den Hoff, 2002). Rate constants (k) describe the rate of movement of 18FDG between compartments. These are assumed to represent specific physiological processes such as blood flow, glucose metabolism and enzyme activity. The transport of 18FDG across the capillary/cellular membrane is accounted for by the rate constants K1 and k2 (forward and reverse transport). The phosphorylation of 18FDG to 18FDG-6-PO4 is represented by k3 and sometimes a small dephosphorylation rate constant, k4, for the dephosphorylation of 18FDG. Ki (K1k3/(k2+k3)) is the net influx rate constant for 18FDG. Certain assumptions have to be made related to the biological system and 18FDG and to the specific experimental procedure employed to make the measurements. The processes of the biological system influencing the kinetic behaviour of 18FDG are assumed to be in steady state, although the 18FDG concentration itself does not have to be in a steady state. The data in dynamic studies are obtained from a series of image frames as described earlier. An IF is measured from either arterial or arterialised venous blood sampling. More recently, noninvasive measurement of arterial activity in the aorta or left ventricle dynamic image data has been validated (Hoekstra et al, 2002).
Nonlinear regression analysis uses an algorithm to derive values for the rate constants and also a blood volume term. It uses a two-tissue compartmental model with an arterial plasma IF and tissue time–activity data (tissue TAC) over 0–60 min to measure Ki. It provides one of the most accurate estimates of tumour glucose use (Dimitrakopoulou-Strauss et al, 2002). In clinical practice, low count statistics and artefacts attributable to patient movement can affect its accuracy.
Patlak graphical analysis requires fewer image frames than NLR but still requires a blood IF (Patlak et al, 1983). A tissue TAC is provided from the dynamic image data and the net metabolic clearance of 18FDG can then be calculated. The rate of uptake from the plasma is given by the slope of the linear portion of the plot. Ki can therefore be derived and the tissue metabolic rate of glucose is calculated. This method assumes that k4=0, that is, irreversible trapping of 18FDG-6-PO4. Patlak analysis has been shown to approach the accuracy of NLR but is computationally simpler and considered less susceptible to image noise (Weber, 2005).
Clinical applications
Tissue differentiation and tumour grading
The initial role of 18FDG PET was in the differentiation between benign and malignant lesions, especially in indeterminate solitary pulmonary nodules (Lowe et al, 1998) with further exploration into grading and prognosis assessment of various tumours (Dimitrakopoulou-Strauss et al, 2001; Shimoda et al, 2007). Standardised uptake values have been shown to be reliable in differentiating between benign and malignant lesions in a number of situations, but in others visual analysis has been reported to be equivalent (Lowe et al, 1994). It is possible that visual analysis may often be sufficient with an experienced interpreter. A number of studies have shown that various forms of kinetic analysis have better specificity (Dimitrakopoulou-Strauss et al, 2002) with improved performance in lesions with borderline SUVs but at the expense of greater scan acquisition and analysis complexity. There is less evidence on the use of PET to determine grade of malignancy, with few dynamic studies reported. However, there is some evidence that 18FDG PET correlates with tumour grade and infers prognosis in some tumour types, examples having been reported in bone, breast and brain tumours, among others (Dimitrakopoulou-Strauss et al, 2001; Borbely et al, 2006; Shimoda et al, 2007).
Assessment of response to treatment and treatment modification
Several studies have demonstrated the ability to predict clinical or pathological response at an early time point by measuring changes in 18FDG tumour activity and to infer a better prognosis in responders. The results of such studies imply that early changes in tumour metabolism predict subsequent response and correlate with outcome. The optimum time point for assessment is likely to be variable, depending on individual tumours and their speed of response to different treatments with variation in studies reflecting different research questions. Wahl et al (1993) showed a reduction in SUV and net 18FDG influx as early as 8 days after starting chemohormonotherapy for primary breast cancer, which predated changes in size and predicted ultimate pathological response. Similarly, Weber et al (2001) have shown that a greater than 35% reduction in SUV in gastro-oesophageal cancers after one cycle of chemotherapy predicts pathological response and increased time to progression and overall survival. Interim response assessment is also being used after one or two cycles of chemotherapy to inform subsequent management, completing therapy in responding patients but intensifying or changing treatment in nonresponders. This approach has been most extensively investigated in lymphoma (Israel et al, 2004).
There is general agreement that at least semiquantitative techniques are superior to qualitative assessment alone in monitoring treatment response (Young et al, 1999; Hoekstra et al, 2000; Weber, 2005; Shankar et al, 2006) and are probably sufficient for most routine clinical work. These methods may be limited where maximal tumour response has not been achieved or when there is an accompanying inflammatory response associated with 18FDG activity. The careful timing of scans after injection is also crucial to ensure maximal precision between scans.
A number of parameters may be affected by the metabolic changes that occur as a result of therapy, such as plasma clearance of 18FDG secondary to reduced renal function. This can affect the assumptions made in interpretations of data, including that the contribution of nonphosphorylated 18FDG to the total signal can be ignored. This can result in the poorer performance of qualitative and semiquantitative analysis. Weber (2005) suggested that although changes in SUV are generally well correlated with changes in net influx rate (Ki), this may not apply in tumours with relatively low metabolic activity. In this situation, the contribution of nonphosphorylated 18FDG to the total 18FDG signal cannot be neglected, and therapy-induced changes in Ki may correlate less well with changes in SUV. Because of these and other described limitations of semiquantitative techniques, there will remain a role for more complex and invasive dynamic scan acquisitions and kinetic analysis to allow for better discrimination of responders from nonresponders (Hoekstra et al, 2002), particularly in the phase 1 trial setting or when novel tracers measuring different pharmacodynamic end points are being used where less knowledge exists on the kinetics of uptake. Compartmental modelling of dynamic data is often the preferred form of analysis for pharmacokinetic measurements of novel drugs as it allows a detailed measure of the individual kinetic processes involved (Willemsen and Van den Hoff, 2002).
Conclusion
Positron emission tomography has become an important part of the management of cancer. Qualitative and semiquantitative interpretation of static scans is probably sufficient for routine clinical staging purposes. In lesions of uncertain significance, semiquantitative techniques (e.g., SUV) may help guide interpretation, although due to variability in 18FDG avidity between different tumour types, may be limited in discrimination between benign and malignant tissue in an individual case. For the assessment of response to therapy, further studies are required. Although semiquantitative measures usually suffice in the clinic, it may be that the use of dynamic scanning with kinetic analysis is more appropriate for drug development trials and may provide a more accurate assessment of changes in tumour metabolism. This may be worth the additional time and effort to perform as patient treatment becomes more tailored to the individual, based on their response to treatment received so far. However, although kinetic approaches offer improved accuracy and discrimination, they are practically more complex and validation of semiquantitative static or simplified dynamic techniques would be particularly attractive in this role.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Borbely K, Nyary I, Toth M, Ericson K, Gulyas B (2006) Optimization of semi-quantification in metabolic PET studies with 18F-fluorodeoxyglucose and 11C-methionine in the determination of malignancy of gliomas. J Neurol Sci 246: 85–94
Caraco C, Aloj L, Chen LY, Chou JY, Eckelman WC (2000) Cellular release of [18F]2-fluoro-2-deoxyglucose as a function of the glucose-6-phosphatase enzyme system. J Biol Chem 16: 18489–18494
Cook GJR, Fogelman I, Maisey MN (1996) Normal physiological and benign pathological variants of 18-FDG PET scanning: potential for error in interpretation. Semin Nucl Med 26: 308–314
Cook GJR, Lodge MA, Marsden PK, Dynes A, Fogelman I (1999) Non-invasive assessment of skeletal kinetics using 18F-fluoride positron emission tomography: evaluation of image and population derived arterial input functions. Eur J Nucl Med 26: 1424–1429
Dimitrakopoulou-Strauss A, Strauss LG, Heichel T, Wu H, Burger C, Bernd L, Ewerbeck V (2002) The role of quantitative (18)F-FDG PET studies for the differentiation of malignant and benign bone lesions. J Nucl Med 43: 510–518
Dimitrakopoulou-Strauss A, Strauss LG, Schwarzbach M, Burger C, Heichel T, Willeke F, Mechtersheimer G, Lehnert T (2001) Dynamic PET 18F-FDG studies in patients with primary and recurrent soft-tissue sarcomas: impact on diagnosis and correlation with grading. J Nucl Med 42: 713–720
Geworski L, Knoop BO, de Cabejas ML, Knapp WH, Munz DL (2000) Recovery correction for quantitation in emission tomography: a feasibility study. Eur J Nucl Med 27: 161–169
Hoekstra C, Hoekstra O, Stroobants S, Vansteenkiste J, Nuyts J, Smit E, Boers M, Twisk J, Lammertsma A (2002) Methods to monitor response to chemotherapy in non-small cell lung cancer with 18F-FDG PET. J Nucl Med 43: 1304–1309
Hoekstra CJ, Paglianiti I, Hoekstra OS, Smit EF, Postmus PE, Teule GJJ, Lammertsma AA (2000) Monitoring response to therapy in cancer using [18F]-2-fluoro-2-deoxy-D-glucose and positron emission tomography: an overview of different analytical methods. Eur J Nucl Med 27: 731–743
Huang SC (2000) Anatomy of SUV. Standardized uptake value. Nucl Med Biol 27: 643–646
Hunter GJ, Hamberg LM, Alpert NM, Choi NC, Fischman AJ (1996) Simplified measurement of deoxyglucose utilization rate. J Nucl Med 37: 950–955
Israel O, Keidar Z, Bar-Shalom R (2004) Positron emission tomography in the evaluation of lymphoma. Semin Nucl Med 34: 166–179
Kaim AH, Weber B, Kurrer MO, Gottschalk J, Von Schulthess GK, Buck A (2002) Autoradiographic quantification of 18F-FDG uptake in experimental soft-tissue abscesses in rats. Radiology 223: 446–451
Lodge MA, Lucas JD, Marsden PK, Cronin BF, O'Doherty MJ, Smith MA (1999) A PET study of 18FDG uptake in soft tissue masses. Eur J Nucl Med 26: 22–30
Lowe VJ, Fletcher JW, Gobar L, Lawson M, Kirchner P, Valk P, Karis J, Hubner K, Delbeke D, Heiberg EV, Patz EF, Coleman RE (1998) Prospective investigation of positron emission tomography in lung nodules. J Clin Oncol 16: 1075–1084
Lowe VJ, Hoffman JM, DeLong DM, Patz EF, Coleman RE (1994) Semiquantitative and visual analysis of FDG-PET images in pulmonary abnormalities. J Nucl Med 35: 1771–1776
Matthies A, Hickeson M, Cuchiara A, Alavi A (2002) Dual time point 18F-FDG PET for the evaluation of pulmonary nodules. J Nucl Med 43: 871–875
Minn H, Zasadny KR, Quint LE, Wahl RL (1995) Lung cancer: reproducibility of quantitative measurements for evaluating [F-18]-fluoro-2-deoxy-D-glucose uptake at PET. Radiology 196: 167–173
Nakamoto Y, Higashi T, Sakahara H, Tamaki N, Kogire M, Doi R, Hosotani R, Imamura M, Konishi J (2000) Delayed 18F-fluoro-2-deoxy-D-glucose positron emission tomography scan for differentiation between malignant and benign lesions in the pancreas. Cancer 89: 2547–2554
Patlak CS, Blasberg RG, Fenstermacher JD (1983) Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 3: 1–7
Ratib O (2004) PET/CT image navigation and communication. J Nucl Med 45 (Suppl 1): 46s–55s
Shankar LK, Hoffman JM, Bacharach S, Graham MM, Karp J, Lammertsma AA, Larson S, Mankoff DA, Siegel BA, Van den Abbeele A, Yap J, Sullivan D (2006) Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute trials. J Nucl Med 47: 1059–1066
Shimoda W, Hayashi M, Murakami K, Oyama T, Sunagawa M (2007) The relationship between FDG uptake in PET scans and biological behavior in breast cancer. Breast Cancer 14: 260–268
Smith TAD (2001) The rate-limiting step for tumour 18F-fluoro-2-deoxy-D-glucose (FDG) incorporation. Nucl Med Biol 28: 1–4
Sundaram S, Freedman N, Carrasquillo J, Carson J, Whatley M, Libutti S, Sellers D, Bacharach S (2004) Simplified kinetic analysis of tumor 18F-FDG uptake: a dynamic approach. J Nucl Med 45: 1328–1333
Thie JA (2004) Understanding the standardized uptake value, its methods, and implications for usage. J Nucl Med 45: 1431–1434
Wahl RL, Zasadny K, Helvie M, Hutchins GD, Weber B, Cody R (1993) Metabolic monitoring of breast cancer chemohormonotherapy using positron emission tomography: initial evaluation. J Clin Oncol 11: 2101–2111
Weber WA, Ott K, Becker K, Dittler HJ, Helmberger H, Avril NE, Meisetschlager G, Busch R, Siewert JR, Schwaiger M, Fink U (2001) Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol 19: 3058–3065
Weber WA (2005) Use of PET for monitoring cancer therapy and for predicting outcome. J Nucl Med 46: 983–995
Willemsen AT, van den Hoff J (2002) Fundamentals of quantitative PET data analysis. Curr Pharm Des 8: 1513–1526
Yamada S, Kubota K, Kubota R, Ido T, Tamahashi N (1995) High accumulation of fluorine-18-fluorodeoxyglucose in turpentine-induced inflammatory tissue. J Nucl Med 36: 1301–1306
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, Pruim J, Price P (1999) Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. Eur J Cancer 35: 1773–1782
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Castell, F., Cook, G. Quantitative techniques in 18FDG PET scanning in oncology. Br J Cancer 98, 1597–1601 (2008). https://doi.org/10.1038/sj.bjc.6604330
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6604330
Keywords
This article is cited by
-
Use of population input functions for reduced scan duration whole-body Patlak 18F-FDG PET imaging
EJNMMI Physics (2021)
-
Partial-volume correction in dynamic PET-CT: effect on tumor kinetic parameter estimation and validation of simplified metrics
EJNMMI Research (2019)
-
Inhibition of 6-phosphofructo-2-kinase (PFKFB3) suppresses glucose metabolism and the growth of HER2+ breast cancer
Breast Cancer Research and Treatment (2016)
-
STatistically Assigned Response Criteria in Solid Tumors (STARCIST)
Cancer Imaging (2015)
-
Fast growth associated with aberrant vasculature and hypoxia in fibroblast growth factor 8b (FGF8b) over-expressing PC-3 prostate tumour xenografts
BMC Cancer (2010)