Abstract
Lactate dehydrogenase-5 (LDH-5) catalyses the reversible transformation of pyruvate to lactate, having a principal position in the anaerobic cellular metabolism. Induction of LDH-5 occurs during hypoxia and LDH-5 transcription is directly regulated by the hypoxia-inducible factor 1 (HIF1). Serum LDH levels have been correlated with poor prognosis and resistance to chemotherapy and radiotherapy in various neoplastic diseases. The expression, however, of LDH in tumours has never been investigated in the past. In the present study, we established an immunohistochemical method to evaluate the LDH-5 overexpression in tumours, using two novel antibodies raised against the rat muscle LDH-5 and the human LDH-5 (Abcam, UK). The subcellular patterns of expression in cancer cells were mixed nuclear and cytoplasmic. In direct contrast to cancer cells, stromal fibroblasts were reactive for LDH-5 only in a minority of cases. Serum LDH, although positively correlated with, does not reliably reflect the intratumoral LDH-5 status. Lactate dehydrogenase-5 overexpression was directly related to HIF1α and 2α, but not with the carbonic anhydrase 9 expression. Patients with tumours bearing high LDH-5 expression had a poor prognosis. Tumours with simultaneous LDH-5 and HIF1α (or HIF2α) overexpression, indicative of a functional HIF pathway, had a particularly aggressive behaviour. It is concluded that overexpression of LDH-5 is a common event in non-small-cell lung cancer, can be easily assessed in paraffin-embedded material and provides important prognostic information, particularly when combined with other endogenous markers of hypoxia and acidity.
Similar content being viewed by others
Main
Rapid cancer cell proliferation and high metabolic demands, defective structural and functional angiogenesis, as well as irregular spatial relation between cancer cells and stromal vasculature are the principal causes of intratumoral hypoxia (Giatromanolaki and Harris, 2001; Vaupel et al, 2001). Reduced oxygen tension is a well-recognised cause of failure of radiotherapy, as reduced intracellular oxygen levels result in decreased free radical formation during irradiation and consequently to a lower amount of DNA strand breaks (Gray et al, 1953; Brown, 2002). Furthermore, the molecular cascade triggered in the context of cancer cell response to the hypoxic stress establishes an aggressive phenotype with increased metastatic potential and resistance to various apoptotic stimuli (Rofstad, 2000; Harris, 2002).
Glycolysis is a major source for the production of ATP from glucose, which is transformed to pyruvate while NAD+ is reduced to NADH. The fate of the pyruvate molecules produced depends on the oxygen presence. Adequate oxygenation allows the conversion of pyruvate to ATP, water and carbon dioxide, the reversible transformation of which to carbonic acid is catalysed by the enzyme carbonic anhydrase (Opavsky et al, 1996). Lack of oxygen or biochemical defects relevant to the electron transport system or the citric acid cycle restricts ATP production to glycolysis. The glycolytic ATP production will continue as long as NAD+ is available, the availability of which is guaranteed if oxidation of NADH back to NAD+ is feasible. Such an oxidation occurs during the reversible transformation of pyruvate to lactate, a reaction catalysed by lactate dehydrogenase-5 (LDH-5) (Holbrook et al, 1975). Lactate dehydrogenase-5 is one of the five isoenzymes of the LDH, composed of four M-subunits and has the highest efficiency among all other isoenzymes to catalyse pyruvate transformation to lactate. The higher the number of H-subunits the LDH contains, the lower the ability of the enzyme to catalyse the reaction, so that LDH-1 (composed of four H-subunits) favours the conversion of pyruvate to acetyl-CoA that enters into the citric acid (Krebs) cycle. Upregulation of LDH-5 by cancer cells guarantees a strong glycolytic metabolism and reduced dependence of cells in the presence of oxygen. The hypoxia-inducible factors 1α and 2α (HIFαs) are key transcription factors regulating glycolysis and the transcription of both LDH-5 and carbonic anhydrase 9 (CA9) (Firth et al, 1995; Semenza et al, 1996; Ebert and Bunn, 1998; Wykoff et al, 2001).
In the present study, we compared the overexpression of LDH-5 and CA9 in non-small-cell lung cancer (NSCLC). Increased activity of LDH, being linked with intratumoral hypoxia and acidity (Yamagata et al, 1998), should be related with aggressive tumour features as shown in a previous study on CA9 in NSCLC (Giatromanolaki et al, 2001a). As HIFαs regulate glycolysis and LDH transcription, we further assessed the HIF association with LDH expression. The prognostic relevance of LDH in comparison with CA9 and HIFαs was also assessed.
Materials and methods
Archival paraffin-embedded biopsy material from 76 primary squamous cell lung carcinomas and 36 lung adenocarcinomas were retrieved, and 3 μm tissue sections were cut on slides (Department of Pathology, University of Oxford, UK). All patients had early operable cancer (T1,2–N0,1 stage) and were treated with surgery alone. Histological diagnosis, grading and N-stage were performed on haematoxylin–eosin-stained sections.
A total of 87 patients were male and 25 female, their ages ranging from 35 to 74 years (median 63). Survival data were available for all 104 patients. At the time of analysis, 54 patients out of 112 were dead, while the median follow-up of surviving patients was 57 months (range 22–83 months).
Cancer tissue samples examined were chosen from the tumour periphery, so that normal lung was included. Specific conditions around the tumour (hypoxia, acidity, high growth factor concentration) could have affected the LDH-5 expression status in the adjacent lung. To avoid such a bias, we further assessed the expression of LDH-5 protein in: (i) 10 tissue samples containing normal alveolar and bronchial tissue obtained from autopsies and (ii) 10 samples from apparently normal lung (located away from the tumour) obtained from patients who underwent pneumonectomy. These samples were retreived from the archives of the department of Pathology, Democritus University of Alexandroupolis, Greece.
LDH-5 immunohistochemistry
The sheep polycloncal ab9002 (Abcam, Cambridge, UK) raised against human LDH-5 purified from human placenta, and the goat polyclonal ab7639 (Abcam, Cambridge, UK) raised against rabbit muscle lactate dehydrogenase were used for immunohistochemistry (Zaman et al, 1999). Ab9002 is an IgG fraction, the identity of which was confirmed by double diffusion against purified LDH-5 and a known anti-human LDH-5. Specificity has been demonstrated by Western blot against liver cell lysate. Ab9002 is available in liquid form, in glycine-buffered saline pH 7.4, 0.1% sodium azide, 0.1% EACA, 0.01% benzamidine and 1 mM EDTA. Ab7639 is an IgG fraction antibody purified from monospecific antiserum by a multistep process including delipidation, salt fractionation and ion exchange chromatography followed by extensive dialysis against a buffer composed of 0.15 M Nacl, 0.01% sodium azide at pH 7.2. Ab7639 is available in liquid form, in protease-free 10 mg ml−1 bovin serum albumin BSA IgG and 0.01% gentamicin sulphate.
A modified streptavidin technique was used for immunohistochemistry. Following multiple experiments, the optimal concentration of the Ab standardised for immunohistochemistry in paraffin-embedded tissues was 50 μg ml−1 (1 : 200) for both antibodies. Sections were deparaffinised and peroxidase was quenched with methanol and H2O2 3% for 15 min. Microwaving for antigen retrieval was used (3 × 5 min). The primary antibody was applied overnight. Following washing with TBS, sections were incubated with a secondary antibody (Kwik Kit, Cat. No. 404050, Thermo Shandon, Pittsburgh, PA 15275, USA) for 15 min and washed in TBS. Kwik streptavidin peroxidase reagent was applied for 15 min and sections were again washed in TBS. The colour was developed by 15 min incubation with DAB solution and sections were weakly counterstained with haematoxylin. Rat renal tissue obtained from hypoxic kidneys after 20 min ligation of the vessels were used for positive controls. Normal goat and sheep immunoglobulin-G was substituted for primary antibody (ab7639 and ab9001, respectively) at a concentration where immunostaining of control slides gave a faint cytoplasmic staining.
Assessment of HIFα and of CA9 expression
The HIF1α and HIF2α proteins were detected using the ESEE 122 (IgG1 Mab; dilution 1 : 20) and the EP190b (IgG1 Mab; neat) monoclonal antibodies as we previously described (Talks et al, 2000). For the detection of CA9, we used the alkaline phosphatase/antialkaline phosphatase (APAAP) procedure and the mouse monoclonal anti-human CA9 antibody M75 (Pastorek et al, 1994). The immunohistochemical technique, assessment of expression and identifications of groups of high vs low HIF or CA9 reactivity used have been described in previous studies (Giatromanolaki et al, 2001a, 2001b). Assessment was based on the percentage of cancer cells with strong cytoplasmic/nuclear HIFα reactivity and of membrane CA9 expression.
Immunohistochemistry for angiogenesis and angiogenic factor expression
The LDH expression was further analysed in comparison with the microvessel density and the expression of angiogenic factors vascular endothelial growth factor (VEGF; VG1 MoAb, University of Oxford, UK), thymidine phosphorylase (TP; PGF-44c, University of Oxford, UK) and basic-Fibroblast growth factor (bFGF) and with the bek-bFGF-receptor (Santa Cruz Biotechnology, USA). The activated VEGF/KDR expressing vascular density was also assessed using the 11B5 Moab (Texas University, USA). Details on the immunohistochemistry and assessment of these parameters have been reported in previous studies of ours (Koukourakis et al, 2000; Giatromanolaki et al, 2000).
Comparison of serum LDH vs immunohistochemistry
In an additional cohort of 33 patients, the LDH serum levels were prospectively assessed with standard biochemical assays. Serum samples were taken immediately before bronchoscopic biopsy (11 patients with inoperable stage IIIb NSCLC) or on the day of operation (22 patients with stage II/IIIa NSCLC who underwent partial or total pneumonectomy). Lactate dehydrogenase serum levels were further assessed 8 days after biopsy or surgery, respectively. Biopsy or surgical material was formalin-fixed and paraffin-embedded, while LDH immunohistochemistry was performed and assessed, the pathologists being blinded to the results of LDH biochemistry. In this way, we could assess the correlation between serum and tissue LDH and, furthermore, we could study the effect of surgery or biopsy on serum LDH levels. The normal levels of serum LDH in our laboratory are <450 IU l−1. Lactate dehydrogenase levels higher than this value were considered as abnormally high.
Statistical analysis
Statistical analysis and graphs were performed using the GraphPad Prism® 2.01 and the Instat® 3.0 packages (San Diego California USA, www.graphpad.com). The χ2 t-test, Fisher's exact t-test or the unpaired two-tailed t-test was used for testing relationships between categorical tumour variables, as appropriate. Linear regression analysis was used to test the relationship between continuous variables. Survival curves were plotted using the method of Kaplan and Meier, and the log-rank test was used to determine statistical differences between life tables. A Cox proportional hazard model was used to assess the effects of patient and tumour variables on overall survival. All P-values are two sided and P-values <0.05 were used for significance.
Results
LDH-5 expression patterns
Normal lung cells (bronchial and alveolar) away from the tumour were unreactive for LDH-5, while positive staining was occasionally noted in normal lung adjacent to the tumour. Chondrocytes of entrapped cartilage showed nuclear patterns of expression. The same patterns of staining were obtained with both antibodies used.
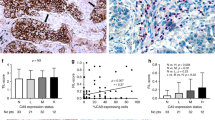
Immunohistochemical expression of LDH-5 in cancer cells revealed both cytoplasmic and nuclear staining patterns. Nuclear expression was usually accompanied by strong cytoplasmic expression of LDH-5, while predominantly cytoplasmic expression was noted in a subset of tumours (Figure 1a,b). Linear regression analysis between the percentage of cells with LDH-5 expression obtained with the ab9002 and ab7639 showed a highly significant correlation (P<0.0001, r=0.92). All subsequent analyses performed were based on the results obtained with the ab9002 (anti-human LDH-5). The median percentage of cells with cytoplasmic LDH-5 expression was 80 (range 0–100; mean 68, s.d. 31). The median percentage of cancer cells with nuclear LDH expression was 10 (0–100; mean 30, s.d. 37). Linear regression analysis revealed a highly significant association between the percentage of cells with cytoplasmic and with nuclear expression (P<0.0001, r=0.64).
Stroma fibroblasts and vessels were also stained for LDH-5 in 24 out of 112 of the cases analysed, the patterns being predominantly cytoplasmic and to a lesser extent nuclear. In all these 24 cases, extensive cancer cell LDH-5 expression was also present. These staining patterns were obtained with both antibodies used.
Definition of cutoff points
Cases with cytoplasmic LDH-5 reactivity higher than the median (>80%) were grouped as bearing high LDH-5 reactivity (45 cases). As this cutoff point was very high due to the frequent and abundant LDH-5 overexpression in NSCLC, the remaining cases were divided into two groups of low (0–50%; 47 cases) and medium (51–80%; 30 cases) LDH-5 reactivity.
Using the median percentage of cells with nuclear patterns of LDH expression,two groups of cases with low vs high LDH-5 nuclear reactivity were defined (low: 0–10% of cells with nuclear LDH-5 expression, 59 cases; high: >10% of cells with nuclear LDH-5 expression, 53 cases).
Lactate dehydrogenase association with histopathological variables
No association of cytoplasmic LDH-5 expression with histology (adenocarcinomas vs squamous cell carcinomas) or histological differentiation was noted. High cytoplasmic LDH-5 expression was more frequent in advanced T-stage (P=0.10) and nodal involvement (P=0.15), but the difference was not statistically significant. Expression of LDH-5 was independent of the degree of necrosis. Similarly, nuclear patterns of LDH-5 expression did not relate to any of the histopathological variables examined.
Lactate dehydrogenase association with HIFαs and CA9
Linear regression analysis between the percentage of cells with cytoplasmic LDH-5 expression and the percentage of cells positive for HIF1α and HIF2α revealed a significant positive correlation (P<0.0001, r2=0.41 and P=0.03, r2=0.20, respectively). No association with CA9 expression was noted (P=0.72, r=0.03). Similar analysis using the percentage of cells with nuclear LDH reactivity did not show any association.
Using categorical variable analysis, high cytoplasmic and high nuclear LDH-5 expression were significantly linked with high HIF1α expression, but not with HIF2α or CA9 expression (Table 1).
Lactate dehydrogenase association with angiogenesis
Using LDH-5 and all angiogenic parameters as continuous variables, linear regression analysis showed a significant association of cytoplasmic (but not of nuclear) LDH-5 with most of the angiogenic factors, but not with microvessel density (Table 2). Categorical variable analysis (using groups as defined in previously reported studies (Koukourakis et al, 2000; Giatromanolaki et al, 2000) showed similar results (data not shown). Briefly, a significant association of high LDH-5 cytoplasmic expression with VEGF cytoplasmic expression was noted (P=0.01). Similarly, a significant association of LDH-5 cytoplasmic expression with bFGF and bek-bFGF-receptor expression was confirmed (P=0.004 and 0.05, respectively), while the association with TP expression was marginal (P=0.07). The association of LDH-5 nuclear expression with these variables was less strong and did not reach significance. Lactate dehydrogenase-5 expression was not associated with microvessel density or the activated VEGF/KDR microvessel density (aMVD).
Association of HIFαs with VEGF and CA9
The association among these variables in NSCLCs, in the same material used in the present study, has been extensively analysed in two previous studies (Giatromanolaki et al, 2001a, 2001b). Briefly, VEGF is directly related to HIF1α and HIF2α but not with CA9. A marginal, not significant, association between CA9 expression and HIF1α was also noted (data not shown).
LDH-5 and overall survival
Figure 2 shows the Kaplan–Meier overall survival curves stratified for cytoplasmic LDH-5 (low vs medium vs high) and nuclear (low vs high) LDH-5 expression. A significantly poorer survival was noted in the group of patients with high LDH-5 cytoplasmic and high LDH-5 nuclear reactivity (P=0.03 and 0.02, respectively).
In multivariate analysis using a model that comprised the T-, N-stage, CA9 and HIFαs expression, LDH-5 expression did not reach an independent prognostic significance (P=0.15, t-ratio=1.44), probably due to the close association with HIFαs and angiogenic factor expression. However, the prognostic usefulness of LDH-5 as a marker is better revealed in double stratification with HIFαs. Overexpression of LDH-5 is a marker of a functional HIFα pathway, and as such LDH-5 could enhance the prognostic usefulness of HIFαs. Indeed, HIF1α and 2α overexpression related to poor overall survival only when LDH-5 was also overexpressed (whether cytoplasmic or nuclear). Figures 3a and b show the Kaplan–Meier survival curves according to HIF1α and 2α expression, respectively, stratified for nuclear LDH-5 reactivity. In multivariate analysis, the combination of nuclear LDH-5 and HIF2α expression showed a very strong independent prognostic relevance. Table 3 shows the multivariate analysis in three statistical models and the relative risk.
An additional survival analysis was performed by stratifying for LDH-5 nuclear and CA9 overexpression. Again, upregulation of either of the enzymes was linked with poor survival (Figure 4).
Serum LDH levels vs immunohistochemistry
The median value of LDH serum levels was 410 IU l−1 (range 234–1294). In all, 20 out of 33 patients had LDH serum levels lower than the upper normal LDH value (450 IU l−1), while in 13 the LDH serum levels were higher than the normal. Linear regression analysis between the serum LDH levels and the percentage of cancer cells with cytoplasmic (and/or nuclear) LDH-5 reactivity showed a statistically significant correlation (P=0.01, r=0.41; Figure 5). Categorical analysis showed , however, that the results from the two methods are not overlapping as 10 out of 20 patients with high LDH-5 tissue reactivity had LDH serum levels lower than the highest normal value (Figure 5). The tumour stroma LDH-5 immunohistochemical reactivity was not related with serum LDH levels (data not shown).
In accordance with the previously reported results, in this series of 33 patients a significant association of tissue LDH-5 expression with HIF1α was recorded, while serum LDH levels were not significantly related to HIF1α overexpression (data not shown).
At eight days following biopsy, the mean serum LDH levels were unchanged (592±300 vs 636±317; P=0.78), while a statistically significant drop was noted in patients who underwent surgery (527±329 vs 369±117; P=0.03). The sharp drop of LDH serum levels was more evident in patients with high preoperative LDH serum levels (P=0.0004); Figure 6.
Discussion
Lactate dehydrogenase, assessed in the sera of cancer patients, is considered as a marker of poor postoperative outcome in a large spectrum of neoplastic diseases including pancreatic carcinoma (Tas et al, 2001a), osteosarcoma (Ferrari et al, 2001), renal and testicular carcinoma (Motzer et al, 1999; vonEyben et al, 2001) and melanoma (Hauschild et al, 1999). An increased metastatic potential of nasopharyngeal carcinomas in patients with high LDH serum levels has also been reported (Cheng et al, 1998). Furthermore, clinicopathological studies suggest that high LDH serum levels are linked with radioresistance of head–neck cancer (Brizel et al, 2001), brain primary and metastatic tumours (Kushner et al, 2001; Lutterbach et al, 1999; Lagerwaard et al, 1999). Elevated LDH serum levels are also linked with resistance to and high rate of relapse after chemotherapy in lymphomas (Kondo et al, 2001; Wilder et al, 2001), breast and ovarian cancer (Ryberg et al, 2001; Yuce et al, 2001), as well as in small cell lung cancer (Argiris et al, 2001; Tas et al, 2001b).
The ominous prognostic significance of serum LDH in malignancy could be attributed to several reasons: (i) increased LDH activity results in lactic acid production and acidification of the extracellular water space, which is a very common feature in cancer (Vaupel et al, 1989; Stubbs et al, 2000). Acidic extracellular pH has been shown to activate gelatinase activity and production of cathepsin D, which contribute to an increased cancer cell invasive ability (Rozhin et al, 1994; Martinez-Zagulian et al, 1996). Activation of macrophage-mediated angiogenesis by lactate may also facilitate metastasis (Jensen et al, 1986; Zabel et al, 1996; Murray and Wilson, 2001). Moreover, low pH protects mitochondria from oxidative stress and may account for increased resistance of cancer cells to hypoxia-induced apoptosis (Bronk and Gores, 1991; Nemoto et al, 2000); (ii) increased LDH production by cancer cells can be a direct marker of intratumoral hypoxia (Firth et al, 1995), and therefore a strong marker of tumour resistance to radiotherapy and some chemotherapeutic agents; (iii) as LDH-5 is transcriptionally regulated by HIFαs, high LDH serum levels may reflect an upregulated HIF-molecular cascade (Firth et al, 1995; Semenza et al, 1996; Ebert and Bunn, 1998). Hypoxia inducible factor stabilisation that occurs due to hypoxia or genetic mutations results in the overexpression of a variety of proteins linked to angiogenesis/metastasis, glycolysis and resistance to apoptosis (Semenza, 1998; Akakura et al, 2001; Harris, 2002). In that way, high serum LDH levels may be an indirect marker of HIF-dependent tumour aggressiveness and resistance to cytotoxic regimens (Aebersold et al, 2001; Koukourakis et al, 2001b; Koukourakis et al, 2002).
Serum levels, however, are unlikely to reflect adequately the intratumoral LDH activity, as the bulk of the neoplastic disease or individual variations of LDH clearance are strong confounding factors. On the other hand, biochemical assessment of LDH from tumour tissue extracts (Beasley et al, 2000) give measurements that strongly vary with the amount of the nonmalignant cellular component (stromal and reactive cells) or even with the extent of necrotic tissue that composes the tumour sample used to obtain tissue extracts. In the present study, we showed that immunohistochemical assessment of LDH-5 allows (i) the differential assessment of LDH-5 production by cancer and noncancerous cells of the tumours, (ii) the intensity of LDH-5 activity within cancer cells and (iii) the identification of subcellular patterns of LDH-5 localisation that could be of biological relevance. Immunohistochemistry, therefore, allows the characterisation of the individual cancer cell LDH activity, which cannot be adequately predicted by serum LDH levels or tissue LDH biochemistry. Indeed, comparative analysis of serum LDH levels and immunohistochemistry showed a positive trend and the results between the two methods were not overlapping.
The patterns of LDH-5 subcellular expression by cancer cells were cytoplasmic with a varying degree of nuclear localisation. Lactate dehydrogenase is well known to reside in both the cytoplasm and nuclei (Reddy and Shukla, 2001). In the majority of cases, the tumoral stroma (fibroblasts, vessels and reactive cells) was unreactive for LDH-5, while in a minority of cases extensive cytoplasmic expression of LDH-5 in stromal cells accompanied LDH-5 overexpression by cancer cells. This finding, together with the observation that serum LDH levels correlated positively with cancer cell LDH-5 reactivity but not with stroma reactivity, strongly suggests that high LDH levels found in the sera of cancer patients are mainly of cancer cell origin, while the contribution of stroma cells is minimal. The rapid drop of LDH serum levels 8 days following surgery further confirms that the excess serum LDH levels found in a subset of NSCLC patients is of tumour origin.
We further assessed whether the LDH-5 expression status by cancer cells relates to any of the histological tumour characteristics. In a previous study by Mall et al (2002), serum LDH levels correlated with advanced stage in small cell lung cancer, and similar findings have been reported for ovarian cancer (Yuce et al, 2001). Poor differentiation has also been linked with high LDH levels in squamous cell HNC (Ross et al, 2000). An increased incidence of metastasis in patients with Ewing's sarcoma or nasopharyngeal carcinoma has also been reported to relate with high serum LDH levels (Cheng et al, 1998; Bacci et al, 2000). In our study, overexpression of LDH-5 by cancer cells was more frequent in large tumours or in cases with node involvement, but this was not statistically significant. Tumour histological type and differentiation or the extent of necrosis were not related to LDH-5 expression.
A strong significant association of LDH-5 overexpression with HIF1α and to a lesser extent with HIF2α was noted, which is in full accordance with studies showing that LDH-5 is transcriptionally regulated by the HIFαs (Semenza et al, 1996; Ebert and Bunn, 1998). Lactate dehydrogenase-5 expression patterns were similar to that observed in HIFα immunostaining, in that reactivity when present was diffuse and not around necrotic areas (Giatromanolaki et al, 2001b). This is in direct contrast with the expression of CA9, where its expression was strongly related with the extent of necrosis and most often localised in cancer cell layers proximal to necrotic areas (Giatromanolaki et al, 2001a). Lactate dehydrogenase-5 was not linked with CA9 expression. Although CA9 gene expression is HIFα regulated (Wykoff et al, 2001), the threshold of hypoxia necessary for the transcription of CA9 or LDH-5 may differ (Giatromanolaki et al, 2001a), which could explain the lack of association between LDH and CA9 expression as well as the discordant relation of these proteins with necrosis. Nevertheless, HIFαs and LDH-5 are additionally regulated by oncogenes and cytokines, which may account for their more diffuse expression than CA9.
In some tumours, overexpression of HIF1α was not accompanied by LDH-5 overexpression. This could show a defective HIF1α pathway, or tumour and gene polymorphism differences in the regulation of individual genes. Alterations on the expression or function of molecules involved in the HIF-DNA binding may exist (Ebert and Bunn, 1998; Ema et al, 1999). On the other hand, the overexpression of LDH in a minority of cases with no HIFα reactivity may indicate hypoxia independent pathways of LDH-5 transcriptional regulation, for example, c-myc activation (Shim et al, 1997).
Combined assessment of HIF1α with LDH-5 expression could therefore predict for an intact or defective HIF pathway. This hypothesis was tested in the survival analysis performed in the present study. In a previous study we showed that HIFαs overexpression is linked with poor postoperative outcome in NSCLC. In the present study LDH-5 overexpression was similarly linked with poor prognosis. Double stratification analysis showed that HIF1α and HIF2α overexpression defined poor prognosis only when LDH-5 was overexpressed. This observation suggests that combined HIFα and LDH-5 assessment is a more potent prognostic tool, reflecting the downstream programme regulated by HIF as being a profile mediating poor prognosis. Further analysis of LDH-5 together with CA9 expression revealed that overexpression of either of the enzymes is independently linked with poor survival. Acidification of the tumour environment expected in both conditions explains in part such an aggressive tumour behaviour (Rozhin et al, 1994; Martinez-Zagulian et al, 1996; Stubbs et al, 2000).
We further examined the association of LDH-5 expression with angiogenesis and activation of various angiogenic pathways. Although LDH was not related to the microvessel density, a significant association of LDH-5 with VEGF, bFGF and to TP expression was noted. Vascular endothelial growth factor coexpression with LDH was expected not only due to the HIFα dependent regulation of both molecules, but also due to a direct effect of acidosis on VEGF upregulation (Fukumura et al, 2001; Shi et al, 2001). In two previous studies, using the same material herein analysed, we showed that VEGF is directly associated with the expression of HIF1α and HIF2α, while CA9 expression was marginally associated with HIF1α (Giatromanolaki et al, 2001b, c). A significant association between LDH and VEGF levels in the pleural fluid of patients with various diseases has been reported (Cheng et al, 1999). The strong association of LDH with bFGF activated pathway, however, may be due to HIF-independent reasons. For example, acidification of the tumour environment by lactate may account for bFGF overexpression (D'Arcangelo et al, 2000). On the other hand, a direct effect of bFGF on LDH cellular levels has been recently shown by Riera et al (2002).
The present study provides the basis for the immunohistochemical assessment of LDH in tumoral tissues. Lactate dehydrogenase immunohistochemistry can better predict the cancer cell LDH activity than serum LDH or biochemistry on tissue extracts. Lactate dehydrogenase cancer cell overexpression related to HIFα overexpression, and the abundant expression of various angiogenic factors. While LDH cancer cell expression represents an important tool to predict aggressive tumour behavior, it becomes a more powerful prognostic tool when combined with HIFα expression. Downregulated LDH on the background of HIFα overexpression allows the identification of a subgroup of tumours with a less aggressive pattern of gene expression regulated by HIF. This may prove to be of importance as therapeutic strategies targeting HIF activity become available (Kung et al, 2000). As endogenous markers of hypoxia have recently focused attention as predictors of response to radiotherapy and chemotherapy (Aebersold et al, 2001; Koukourakis et al, 2001a, 2001b, 2002), combined HIF/LDH analysis may become of value. The expression of LDH was independent of another enzyme involved in tumour acidity and hypoxia, namely CA9, although expression of either of the enzymes related to an ominous prognosis. It seems that LDH, CA9 and HIFαs immunohistochemistry can reliably predict tumour aggressiveness related to acidity and hypoxia, although this requires further investigation.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Aebersold DM, Burri P, Beer KT, Laissue J, Djonov V, Greiner RH, Semenza GL (2001) Expression of hypoxia-inducible factor-1alpha: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res 61: 2911–2916
Akakura N, Kobayashi M, Horiuchi I, Suzuki A, Wang J, Chen J, Niizeki H, Kawamura K, Hosokawa M, Asaka M (2001) Constitutive expression of hypoxia-inducible factor-1alpha renders pancreatic cancer cells resistant to apoptosis induced by hypoxia and nutrient deprivation. Cancer Res 61: 6548–6554
Argiris A, Murren JR (2001) Staging and clinical prognostic factors for small-cell lung cancer. Cancer J 7: 437–447
Bacci G, Ferrari S, Bertoni F, Rimondini S, Longhi A, Bacchini P, Forni C, Manfrini M, Donati D, Picci P (2000) Prognostic factors in nonmetastatic Ewing's sarcoma of bone treated with adjuvant chemotherapy: analysis of 359 patients at the Istituto Ortopedico Rizzoli. J Clin Oncol 18: 4–11
Beasley NJ, Leek R, Alam M, Turley H, Cox GJ, Gatter K, Millard P, Fuggle S, Harris AL (2000) Hypoxia-inducible factors HIF-1alpha and HIF-2alpha in head and neck cancer relationship to tumor biology and treatment outcome in surgically resected patients. Cancer Res 62: 2493–2497
Brizel DM, Schroeder T, Scher RL, Walenta S, Clough RW, Dewhirst MW, Mueller-Klieser W (2001) Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys 51: 349–353
Bronk SF, Gores GJ (1991) Acidosis protects against lethal oxidative injury of liver sinusoidal endothelial cells. Hepatology 14: 150–157
Brown JM (2002) Tumor microenvironment and the response to anticancer therapy. Cancer Biol Ther 1: 453–458
Cheng D, Rodriguez RM, Perkett EA, Rogers J, Bienvenu G, Lappalainen U, Light RW (1999) Vascular endothelial growth factor in pleural fluid. Chest 116: 760–765
Cheng SH, Jian JJ, Tsai SY, Chan KY, Yen LK, Chu NM, Tan TD, Tsou MH, Huang AT (1998) Prognostic features and treatment outcome in locoregionally advanced nasopharyngeal carcinoma following concurrent chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys 41: 755–762
D'Arcangelo D, Facchiano F, Barlucchi LM, Melillo G, Illi B, Testolin L, Gaetano C, Capogrossi MC (2000) Acidosis inhibits endothelial cell apoptosis and function and induces basic fibroblast growth factor and vascular endothelial growth factor expression. Circ Res 86: 312–318
Ebert BL, Bunn HF (1998) Regulation of transcription by hypoxia requires a multiprotein complex that includes hypoxia-inducible factor 1, an adjacent transcription factor, and p300/CREB binding protein. Mol Cell Biol 18: 4089–4096
Ema M, Hirota K, Mimura J, Abe H, Yodoi J, Sogawa K, Poellinger L, Fujii-Kuruyama Y (1999) Molecular mechanisms of transcription activation by HLF and HIF1a in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO 18: 1905–1914
Ferrari S, Bertoni F, Mercuri M, Picci P, Giacomini S, Longhi A, Bacci G (2001) Predictive factors of disease-free survival for non-metastatic osteosarcoma of the extremity: an analysis of 300 patients treated at the Rizzoli Institute. Ann Oncol 12: 1145–1150
Firth JD, Ebert BL, Ratcliffe PJ (1995) Hypoxic regulation of lactate dehydrogenase A Interaction between hypoxia-inducible factor 1 and cAMP response elements. J Biol Chem 270: 21021–21027
Fukumura D, Xu L, Chen Y, Gohongi T, Seed B, Jain RK (2001) Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res 61: 6020–6024
Giatromanolaki A, Koukourakis MI, Sivridis E, O'Byrne K, Cox G, Thorpe PE, Gatter KC, Harris AL (2000) Coexpression of MUC1 glycoprotein with multiple angiogenic factors in non-small cell lung cancer suggests coactivation of angiogenic and migration pathways. Clin Cancer Res 6: 1917–1921
Giatromanolaki A, Harris AL (2001) Tumour hypoxia, hypoxia signaling pathways and hypoxia inducible factor expression in human cancer. Anticancer Res 21: 4317–4324
Giatromanolaki A, Koukourakis MI, Sivridis E, Pastorek J, Wykoff CC, Gatter KC, Harris AL (2001a) Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res 61: 7992–7998
Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Talks K, Pezzella F, Gatter KC, Harris AL (2001b) Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer 85: 881–890
Gray LH, Conger AD, Ebert M (1953) The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol 26: 683
Harris AL (2002) Hypoxia: a key regulatory factor in tumour growth. Nat Rev Cancer 2: 38–47
Hauschild A, Michaelsen J, Brenner W, Rudolph P, Glaser R, Henze E, Christophers E (1999) Prognostic significance of serum S100B detection compared with routine blood parameters in advanced metastatic melanoma patients. Melanoma Res 9: 155–161
Holbrook JJ, Liljas A, Steindel SJ, Rossman MG (1975) Lactate dehydrogenase. In The Enzymes Boyer PD (ed.),, Vol. XI, 3rd edn, pp. 191–292. New York: Academic Press
Jensen JA, Hunt TK, Scheuenstuhl H, Banda MJ (1986) Effect of lactate, pyruvate, and pH on secretion of angiogenesis and mitogenesis factors by macrophages. Lab Invest 54: 574–578
Kondo E, Ogura M, Kagami Y, Taji H, Miura K, Takeuchi T, Maeda S, Asakura S, Suzuki R, Nakamura S, Morishima Y (2001) Assessment of prognostic factors in follicular lymphoma patients. Int J Hematol 73: 363–368
Koukourakis MI, Giatromanolaki A, Sivridis E, Simopoulos K, Pastorek J, Wykoff CC, Gatter KC, Harris AL (2001a) Hypoxia-regulated carbonic anhydrase-9 (CA9) relates to poor vascularization and resistance of squamous cell head and neck cancer to chemoradiotherapy. Clin Cancer Res 7: 3399–3403
Koukourakis MI, Giatromanolaki A, Sivridis E, Simopoulos C, Turley H, Talks K, Gatter KC, Harris AL (2002) Hypoxia-inducible factor (HIF1A and HIF2A), angiogenesis, and chemoradiotherapy outcome of squamous cell head-and-neck cancer. Int J Radiat Oncol Biol Phys 53: 1192–1202
Koukourakis MI, Giatromanolaki A, Skarlatos J Corti L, Blandamura S, Piazza M, Gatter KC, Harris AL (2001b) Hypoxia inducible factor (HIF-1a and HIF-2a) expression in early esophageal cancer and response to photodynamic therapy and radiotherapy. Cancer Res 61: 1830–1832
Koukourakis MI, Giatromanolaki A, Thorpe PE, Brekken RA, Sivridis E, Kakolyris S, Georgoulias V, Gatter KC, Harris AL (2000) Vascular endothelial growth factor/KDR activated microvessel density versus CD31 standard microvessel density in non-small cell lung cancer. Cancer Res 60: 3088–3095
Kung AL, Wang S, Klco JM, Kaelin WG, Livingston DM (2000) Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nat Med 6: 1335–1340
Kushner BH, Wolden S, LaQuaglia MP, Kramer K, Verbel D, Heller G, Cheung NK (2001) Hyperfractionated low-dose radiotherapy for high-risk neuroblastoma after intensive chemotherapy and surgery. J Clin Oncol 19: 2821–2828
Lagerwaard FJ, Levendag PC, Nowak PJ, Eijkenboom WM, Hanssens PE, Schmitz PI (1999) Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys 43: 795–803
Lutterbach J, Weigel P, Guttenberger R, Hinkelbein W (1999) Accelerated hyperfractionated radiotherapy in 149 patients with glioblastoma multiforme. Radiother Oncol 53: 49–52
Mall JW, Schwenk W, Philipp AW, Meyer-Kipker C, Mall W, Muller J, Pollmann C (2002) Serum vascular endothelial growth factor levels correlate better with tumour stage in small cell lung cancer than albumin, neuron-specific enolase or lactate dehydrogenase. Respirology 7: 99–102
Martinez-Zaguilan R, Seftor EA, Seftor RE, Chu YW, Gillies RJ, Hendrix MJ (1996) Acidic pH enhances the invasive behavior of human melanoma cells. Clin Exp Metast 14: 176–186
Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J (1999) Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 17: 2530–2540
Murray B, Wilson DJ (2001) A study of metabolites as intermediate effectors in angiogenesis. Angiogenesis 4: 71–77
Nemoto S, Takeda K, Yu ZX, Ferrans VJ, Finkel T (2000) Role for mitochondrial oxidants as regulators of cellular metabolism. Mol Cell Biol 20: 7311–7318
Opavsky R, Pastorekova S, Zelnik V, Gibadulinova A, Stanbridge EJ, Zavada J, Kettmann R, Pastorec J (1996) Human MN/CA9 gene, a novel member of the carbonic anhydrase family: structure and exon to protein domain relationships. Genomics 33: 480–487
Pastorek P, Pastorekova S, Callebaut I, Mornon JP, Zelnik V, Opavsky R, Zat'ovicova M, Liao S, Portetelle D, Stanbridge EJ, Zavada J, Burny A, Kettmann R (1994) Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene 9: 2877–2888
Reddy MA, Shukla SD (2001) Nuclear activation and translocation of mitogen-activated protein kinases modulated by ethanol in embryonic liver cells. Biochim Biophys Acta 1497: 271–278
Riera MF, Meroni SB, Schteingart HF, Pellizzari EH, Cigorraga SB (2002) Regulation of lactate production and glucose transport as well as of glucose transporter 1 and lactate dehydrogenase A mRNA levels by basic fibroblast growth factor in rat Sertoli cells. J Endocrinol 173: 335–343
Rofstad EK (2000) Microenvironment-induced cancer metastasis. Int J Radiat Biol 76: 589–605
Rozhin J, Sameni M, Ziegler G, Sloane BF (1994) Pericellular pH affects distribution and secretion of cathepsin B in malignant cells. Cancer Res 54: 6517–6525
Ross CD, Gomaa MA, Gillies E, Juengel R, Medina JE (2000) Tumor grade, microvessel density, and activities of malate dehydrogenase, lactate dehydrogenase, and hexokinase in squamous cell carcinoma. Otolaryngol Head Neck Surg 122: 195–200
Ryberg M, Nielsen D, Osterlind K, Skovsgaard T, Dombernowsky P (2001) Prognostic factors and long-term survival in 585 patients with metastatic breast cancer treated with epirubicin-based chemotherapy. Ann Oncol 12: 81–87
Semenza GL (1998) Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev 8: 588–594
Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A (1996) Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem 271: 32529–32537
Shi Q, Le X, Wang B, Abbruzzese JL, Xiong Q, He Y, Xie K (2001) Regulation of vascular endothelial growth factor expression by acidosis in human cancer cells. Oncogene 20: 3751–3756
Shim H, Dolde C, Lewis BC, Wu CS, Dang G, Jungmann RA, Dalla-Favera R, Dang CV (1997) c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci U S A. 94: 6658–6663
Stubbs M, McSheehy PM, Griffiths JR, Bashford CL (2000) Causes and consequences of tumour acidity and implications for treatment. Mol Med Today 6: 15–19
Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL (2000) The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol 57: 411–421
Tas F, Aydiner A, Demir C, Topuz E (2001b) Serum lactate dehydrogenase levels at presentation predict outcome of patients with limited-stage small-cell lung cancer. Am J Clin Oncol 24: 376–378
Tas F, Aykan F, Alici S, Kaytan E, Aydiner A, Topuz E (2001a) Prognostic factors in pancreatic carcinoma: serum LDH levels predict survival in metastatic disease. Am J Clin Oncol 24: 547–550
Vaupel P, Kallinowski F, Okunieff P (1989) Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res 49: 6449–6465
Vaupel P, Thews O, Hoeckel M (2001) Treatment resistance of solid tumors: role of hypoxia and anemia. Med Oncol 18: 243–259
von Eyben FE, Madsen EL, Blaabjerg O, Petersen PH, von der Maase H, Jacobsen GK, Rorth M (2001) Serum lactate dehydrogenase isoenzyme 1 and relapse in patients with nonseminomatous testicular germ cell tumors clinical stage I. Acta Oncol 40: 536–540
Wilder RB, Rodriguez MA, Ha CS, Pro B, Hess MA, Cabanillas F, Cox JD (2001) Bulky disease is an adverse prognostic factor in patients treated with chemotherapy comprised of cyclophosphamide, doxorubicin, vincristine, and prednisone with or without radiotherapy for aggressive lymphoma. Cancer 91: 2440–2446
Wykoff CC, Beasley NJP, Watson PH, Turner KJ, Pastorek J, Wilson GD, Turley H, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL (2001) Hypoxia inducible regulation of tumor associated carbonic anhydrases. Cancer Res 60: 7075–7083
Yamagata M, Hasuda K, Stamato T, Tannock IF (1998) The contribution of lactic acid to acidification of tumours: studies of variant cells lacking lactate dehydrogenase. Br J Cancer 77: 1726–1731
Yuce K, Baykal C, Genc C, Al A, Ayhan A (2001) Diagnostic and prognostic value of serum and peritoneal fluid lactate dehydrogenase in epithelial ovarian cancer. Eur J Gynaecol Oncol 22: 228–232
Zabel DD, Feng JJ, Scheuenstuhl H, Hunt TK, Hussain MZ (1996) Lactate stimulation of macrophage-derived angiogenic activity is associated with inhibition of poly(ADP-ribose) synthesis. Lab Invest 74: 644–649
Zaman K, Ryu H, Hall D, O'Donovan K, Lin KI, Miller MP, Marquis JC, Baraban JM, Semenza GL, Ratan RR (1999) Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, p21(waf1/cip1), and erythropoietin. J Neurosci 19: 9821–9830
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Koukourakis, M., Giatromanolaki, A., Sivridis, E. et al. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer 89, 877–885 (2003). https://doi.org/10.1038/sj.bjc.6601205
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6601205
Keywords
This article is cited by
-
Targeting metabolism by B-raf inhibitors and diclofenac restrains the viability of BRAF-mutated thyroid carcinomas with Hif-1α-mediated glycolytic phenotype
British Journal of Cancer (2023)
-
Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions
Signal Transduction and Targeted Therapy (2023)
-
The role of circulating biomarkers in predicting the 30-day mortality of immune checkpoint inhibitors-related myocarditis: a retrospective cohort study
Internal and Emergency Medicine (2023)
-
Lung immune prognostic index‑based nomogram for recurrence of hepatocellular carcinoma after postoperative adjuvant TACE
Journal of Cancer Research and Clinical Oncology (2023)
-
Elesclomol: a copper ionophore targeting mitochondrial metabolism for cancer therapy
Journal of Experimental & Clinical Cancer Research (2022)