Abstract
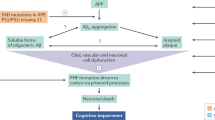
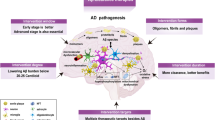
Disease-modifying treatments for Alzheimer disease (AD) have focused mainly on reducing levels of amyloid-β (Aβ) in the brain. Some compounds have achieved this goal, but none has produced clinically meaningful results. Several methodological issues relating to clinical trials of these agents might explain this failure; an additional consideration is that the amyloid cascade hypothesis—which places amyloid plaques at the heart of AD pathogenesis—does not fully integrate a large body of data relevant to the emergence of clinical AD. Importantly, amyloid deposition is not strongly correlated with cognition in multivariate analyses, unlike hyperphosphorylated tau, neurofibrillary tangles, and synaptic and neuronal loss, which are closely associated with memory deficits. Targeting tau pathology, therefore, might be more clinically effective than Aβ-directed therapies. Furthermore, numerous immunization studies in animal models indicate that reduction of intracellular levels of tau and phosphorylated tau is possible, and is associated with improved cognitive performance. Several tau-related vaccines are in advanced preclinical stages and will soon enter clinical trials. In this article, we present a critical analysis of the failure of Aβ-directed therapies, discuss limitations of the amyloid cascade hypothesis, and suggest the potential value of tau-targeted therapy for AD.
Key Points
-
The efficacy of amyloid-β (Aβ) immunization observed in animal models of Alzheimer disease (AD) is not reflected in patients with this disease
-
Immunization against Aβ in patients with mild-to-moderately severe AD reduced levels of Aβ peptides, but failed to improve cognitive function
-
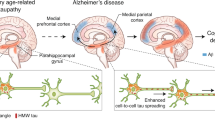
Levels of the microtubule-associated protein tau, hyperphosphorylated tau, and the number of neurofibrillary tangles, synapses and neurons (but not Aβ load) correlate strongly with cognition in AD
-
These findings and results of clinical trials suggest that Aβ might not be the best therapeutic target in AD
-
Tau immunotherapy has been shown to reduce tau pathology and improve cognitive deficits in animal models of AD
-
The amyloid cascade theory of AD pathogenesis remains a useful but unproven hypothesis that should be revised to emphasize the crucial role of tau as a candidate target for AD therapy
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hardy, J. A. & Higgins, G. A. Alzheimer's disease: the amyloid cascade hypothesis. Science 256, 184–185 (1992).
Ferreira, S. T. & Klein, W. L. The Aβ oligomer hypothesis for synapse failure and memory loss in Alzheimer's disease. Neurobiol. Learn. Mem. 96, 529–543 (2011).
Giacobini, E. & Becker, R. E. One hundred years after discovery of Alzheimer's disease. A turning point for therapy? J. Alzheimers Dis. 12, 37–52 (2007).
Becker, R. E. & Greig, N. H. Why so few drugs for Alzheimer's disease? Are methods failing drugs? Curr. Alzheimer Res. 7, 27–35 (2010).
Becker, R. E., Greig, H. H. & Giacobini, E. Why do so many drugs for Alzheimer's disease fail in development? Time for new methods and new practices? J. Alzheimers Dis. 15, 303–325 (2008).
Becker, R. E. & Greig, H. H. Increasing the success rate for Alzheimer's disease drug discovery and development. Expert Opin. Drug Discov. 4, 367–370 (2012).
Hardy, J. Testing times for the “amyloid cascade hypothesis”. Neurobiol. Aging 6, 1073–1074 (2002).
Hardy, J. Alzheimer disease: the amyloid cascade hypothesis: an update and reappraisal. J. Alzheimers Dis. 9, 151–153 (2006).
Xia, W., Wong, S. T., Hanlon, E. & Morin, P. γ-Secretase modulator in Alzheimer's disease: shifting the end. J. Alzheimers Dis. 31, 685–696 (2012).
Green, R. C. et al. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA 302, 2557–2564 (2009).
Henley, D. B., May, P. C., Dean, R. A. & Siemers, E. R. Development of semagacestat (LY450139), a functional gamma-secretase inhibitor, for the treatment of Alzheimer's disease. Exp. Opin. Pharmacother. 10, 1657–1674 (2009).
Eli Lilly and Company. Lilly halts development of semagacestat for Alzheimer's disease based on preliminary results of phase III clinical trials. Eli Lilly and Company[online], (2010).
Coric, V. et al. Safety and tolerability of the γ-secretase inhibitor avagacestat in a phase 2 study of mild to moderate Alzheimer disease. Arch. Neurol. 69, 1430–1440 (2012).
Atwal, J. K. et al. A therapeutic antibody targeting BACE1 inhibits amyloid-β production in vivo. Sci. Transl. Med. 3, 84ra43 (2011).
US National Library of Medicine. ClinicalTrials.gov[online], (2013).
US National Library of Medicine. ClinicalTrials.gov[online], (2013).
Dodel, R. et al. Intravenous immunoglobulin for treatment of mild-to-moderate Alzheimer's disease: a phase 2, randomized, double-blind, placebo-controlled, dose-finding trial. Lancet Neurol. 12, 233–243 (2013).
Buttini, M. et al. Beta-amyloid immunotherapy prevents synaptic degeneration in a mouse model of Alzheimer's disease. J. Neurosci. 40, 9096–9101 (2005).
Gilman, S. M., Koller, M. & Black, R. S. Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial. Neurology 64, 1553–1562 (2005).
Boche, D., Denham, N., Holmes, C. & Nicoll, J. A. Neuropathology after active Aβ42 immunotherapy: implications for Alzheimer's disease pathogenesis. Acta Neuropathol. 120, 369–384 (2010).
Bard, F. et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 6, 916–919 (2000).
Rinne, J. O. et al. 11C-PIB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 4, 363–372 (2010).
Blennow, K. et al. Effect of immunotherapy with bapineuzumab on cerebrospinal fluid biomarkers levels in patients with mild to moderate Alzheimer disease. Arch. Neurol. 69, 1002–1010 (2012).
Business Wire. Pfizer announces topline results of first of four studies in bapineuzumab phase 3 program. Business Wire[online], (2012).
Samadi, H. & Sultzer, D. Solanezumab for Alzheimer's disease. Expert Opin. Biol. Ther. 11, 787–798 (2011).
Dodart, J. C. et al. Immunization reverses memory deficits without reducing brain Aβ burden in Alzheimer's disease model. Nat. Neurosci. 5, 452–457 (2002).
Eli Lilly and Company. Lilly announces detailed results of phase 3 Solanezumab EXPEDITIONS studies following a presentation of the independent analyses by the Alzheimer's Disease Cooperative Study (ADCS). Eli Lilly and Company[online], (2012).
Tariot, P. N. Maintaining cognitive function in Alzheimer disease: how effective are current treatments? Alzheimer Dis. Assoc. Disord. 15 (Suppl. 1), S26–S33 (2001).
Giacobini, E. In The Brain Cholinergic System (eds Giacobini, E. & Pepeu, G. C.) 235–264 (Informa HealthCare, 2006).
Mullane, K. & Williams, M. Alzheimer's therapeutics: continued clinical failures question the validity of the amyloid hypothesis—but what lies beyond? Biochem. Pharmacol. 85, 289–305 (2013).
Aisen, P. S. et al. Tramiprosate in mild-to-moderate Alzheimer's disease—a randomized, double-blind, placebo-controlled, multi-centre study (the Alphase Study). Arch. Med. Sci. 7, 102–111 (2011).
Lannfelt, L. et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Aβ as a modifying therapy for Alzheimer's disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 9, 779–786 (2008).
Salloway, S. et al. A phase 2 randomized trial of ELND005, scyllo-inositol, in mild to moderate Alzheimer Disease. Neurology 77, 1253–1262 (2011).
Walker, J. R. et al. Enhanced proteolytic clearance of plasma Aβ by peripherally administered neprilysin does not result in reduced levels of brain Aβ in mice. J. Neurosci. 33, 2457–2464 (2013).
Arriagada, P. V., Growdon, J. H., Hedley-White, E. T. & Hyman, B. T. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology 42, 631–639 (1992).
Giannakopoulos, P. et al. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology 60, 1495–1500 (2003).
Gold, G. et al. Clinical validity of Aβ-protein deposition staging in brain aging and Alzheimer disease. J. Neuropathol. Exp. Neurol. 60, 946–952 (2001).
Gold, G. et al. Clinical validity of Braak neuropathological staging in the oldest-old. Acta Neuropathol. 99, 579–582 (2000).
Terry, R. D. et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30, 572–580 (1991).
Pievani, M., de Haan, W., Wu, T., Seeley, W. W. & Frisoni, G. B. Functional network disruption in degenerative dementias. Lancet Neurol. 10, 829–843 (2011).
LaFerla, F. M. & Green, K. N. Animal models of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2, a006320 (2012).
Desikan, R. S. et al. Amyloid-β-associated clinical decline occurs only in the presence of elevated P-tau. Arch. Neurol. 69, 700–713 (2012).
Corrada, M. M., Berlau, D. & Kawas, C. H. A population-based clinicopathological study in the oldest-old: the 90+ Study. Curr. Alzheimer Res. 9, 709–716 (2012).
Balasubramanian, A. B. et al. Alzheimer disease pathology and longitudinal cognitive performance in the oldest-old with no dementia. Neurol. 79, 915–921 (2012).
Jack, C. R. Jr et al. Brain β-amyloid load approaches a plateau. Neurology 80, 890–896 (2013).
Villemagne, V. L. et al. Amyloid-β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective study. Lancet Neurol. 12, 357–367 (2013).
Doré, V. et al. Cross-sectional and longitudinal analysis of the relationship between Aβ deposition, cortical thickness, and memory in cognitively unimpaired individuals and in Alzheimer's disease. JAMA Neurol. 27, 1–9 (2013).
Knopman, D. S. et al. Brain injury biomarkers are not dependent on β-amyloid in normal elderly. Ann. Neurol. 73, 472–480 (2013).
Marchant, N. L. et al. The aging brain and cognition: contribution of vascular injury and Aβ to mild cognitive dysfunction. JAMA Neurol. 70, 488–495 (2013).
Wirth, M. et al. Alzheimer's disease neurodegenerative biomarkers are associated with decreased cognitive function but not β-amyloid in cognitively normal older individuals. J. Neurosci. 33, 5553–5556 (2013).
Haass, C. & Selkoe, D. J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid β-peptide. Nat. Rev. Mol. Cell. Biol. 8, 101–112 (2007).
Lesné, S. E. et al. Brain amyloid-β oligomers in ageing and Alzheimer's disease. Brain 136, 1383–1398 (2013).
Hardy, J. Has the amyloid cascade hypothesis for Alzheimer's disease been proved? Curr. Alzheimer Res. 3, 71–73 (2006).
Ittner, L. M. & Götz, J. Amyloid-β and tau—a toxic pas de deux in Alzheimer's disease. Nat. Rev. Neurosci. 12, 65–72 (2011).
Karran, E., Mercken, M. & De Strooper, B. The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 10, 698–712 (2011).
Grundke-Iqbal, K. et al. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl Acad. Sci. USA 83, 4913–4917 (1986).
Braak, H., Braak, E., Grundke-Iqbal, I. & Iqbal, K. Occurrence of neuropil threads in the senile human brain and in Alzheimer's disease: a third location of paired helical filaments outside of neurofibrillary tangles and neuritic plaques. Neurosci. Lett. 65, 351–355 (1986).
Khatoon, S., Grundke-Iqbal, I. & Iqbal, K. Brain levels of microtubule-associated protein tau are elevated in Alzheimer's disease: a radioimmuno-slot-blot assay for nanograms of the protein. J. Neurochem. 59, 750–753 (1992).
Alonso, A., Zaidi, T., Novak, M., Grundke-Iqbal, I. & Iqbal, K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc. Natl Acad. Sci. USA 98, 6923–6928 (2001).
Wang, J. Z., Grundke-Iqbal, I. & Iqbal, K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur. J. Neurosci. 25, 59–68 (2007).
Bancher, C. et al. Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer’s disease. Brain Res. 16, 90–99 (1989).
Handoko, M. et al. Correlations of specific amyloid-β oligomers with tau in cerebrospinal fluid from cognitively normal older adults. JAMA Neurol. 70, 594–599 (2013).
Ashe, K. H. & Zahs, K. R. Probing the biology of Alzheimer's disease in mice. Neuron 10, 631–645 (2010).
De Calignon, A. et al. Propagation of tau pathology in a model of early Alzheimer's disease. Neuron 73, 685–697 (2012).
Oddo, S. et al. Reduction of soluble Aβ and tau, but not soluble Aβ alone, ameliorates cognitive decline in transgenic plaques and tangles. J. Biol. Chem. 281, 39413–39423 (2006).
Oddo, S., Billings, J., Kesslak, J. P., Cribbs, D. H. & LaFerla, F. M. Aβ immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron 43, 321–332 (2004).
Nagy, Z. et al. Relative roles of plaques and tangles in the dementia of Alzheimer's disease: correlations using three sets of neuropathological criteria. Dementia 6, 21–31 (1995).
Robertson, E. D. et al. Reducing endogenous tau ameliorates amyloid-β-induced deficits in an Alzheimer's disease mouse model. Science 316, 750–754 (2007).
Rosenmann, H. et al. Tauopathy-like abnormalities and neurologic deficits in mice immunized with neuronal tau protein. Arch. Neurol. 63, 1459–1467 (2006).
Asuni, A. A., Boutajangout, A., Quartermain, D. & Sigurdsson, E. M. Immunotherapy targeting pathological tau conformers in a tangle mouse reduces brain pathology with associated functional improvement. J. Neurosci. 27, 9115–9129 (2007).
Novak, M. Tau vaccine: active immunization with a misfolded tau protein attenuates tau pathology in the transgenic rat model of tauopathy. Alzheimers Dement. 5, P93 (2009).
Bi, M., Ittner, A., Ke, J. D., Götz, J. & Ittner, L. M. Tau-targeted immunization impedes progression of neurofibrillary histopathology in aged P301L tau transgenic mice. PLoS ONE 6, 12–18 (2012).
Chai, X. et al. Passive immunization with anti-tau antibodies in two transgenic models: reduction of tau pathology and delay of disease progression. J. Biol. Chem. 286, 34457–34467 (2012).
Troquier, L. et al. Targeting phospho-Ser422 by active tau immunotherapy in the THY Tau22 mouse model: a suitable therapeutic approach. Curr. Alz. Res. 9, 397–405 (2012).
Novak, M. Tau transgenic rat model and response to tau vaccine. Alzheimers Dement. 6, S118 (2010).
Theunis, C. et al. Protein tau, target for immunotherapy: preclinical evaluation in transgenic mice [abstract]. Neurodegener. Dis. 8, (Suppl. 1, 2011).
Krishnamurthy, P., Gonzales, V., Rajamohamedsait, H. B. & Sigurdsson, E. Immunization with a pseudo-phosphorylated tau epitope clears tau pathology in a mouse model [abstract]. Alzheimers Dement. 7, S481–S482 (2011).
Yoshiyama, Y., Lee, V. M. & Trojanowski, J. Q. Therapeutic strategies for tau mediated neurodegeneration. J. Neurol. Neurosurg. Psychiatry 84, 784–795 (2013).
Hochgräfe, K., Sydow, A. & Mandelkow, E. M. Regulatable transgenic mouse models of Alzheimer disease: onset, reversibility and spreading of tau pathology. FEBS J. 280, 4371–4381 (2013).
Zhang, B. et al. Microtubule-binding drugs offset tau sequestration by stabilizing mictrotubules and reversing fast axonal transport deficits in a tauopathy model. Proc. Natl Acad. Sci. USA 102, 227–231 (2005).
Gold, M. et al. Critical appraisal of the role of davunetide in the treatment of progressive supranuclear palsy. Neuropsychiatr. Dis. Treat. 8, 85–93 (2012).
Jouroukhin, Y. et al. NAP (davenutide) modifies disease progression in a mouse model of severe neurodegeneration: protection against impairments in axonal transport. Neurobiol. Dis. 56, 79–94 (2013).
Götz, J., Ittner, A. & Ittner, L. Tau-targeted treatment strategies in Alzheimer's disease. Br. J. Pharm. 165, 1246–1259 (2012).
Wischick, C. & Staff, R. Challenges in the conduct of disesase-modifying trials in AD: practical experience from a phase 2 trial of tau-aggregation inhibitory therapy. J. Nutr. Health Aging 13, 367–369 (2009).
Macdonald, A. et al. A feasibility and tolerability study of lithium in Alzheimer's disease. Int. J. Geriat. Psychiatry 23, 704–711 (2008).
Tariot, P. N. et al. Chronic divalproex sodium to attenuate agitation and clinical progression of Alzheimer disease. Arch. Gen. Psychiatry 68, 853–861 (2011).
Hu, J. P. et al. Valproate reduces tau phosphorylation via cyclin-dependent kinase 5 and glycogen synthase kinase 3 signaling pathways. Brain Res. Bull. 30, 194–200 (2011).
Glenner, G. G. & Wong, C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 120, 885–890 (1984).
Author information
Authors and Affiliations
Contributions
Both authors made substantial contributions to researching the data for the article, to discussions of the content, to writing the article, and to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
G. Gold is currently a member of an advisory board for AC Immune. E. Giacobini declares no competing interests.
Rights and permissions
About this article
Cite this article
Giacobini, E., Gold, G. Alzheimer disease therapy—moving from amyloid-β to tau. Nat Rev Neurol 9, 677–686 (2013). https://doi.org/10.1038/nrneurol.2013.223
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2013.223
This article is cited by
-
Rise and fall of peroxisomes during Alzheimer´s disease: a pilot study in human brains
Acta Neuropathologica Communications (2023)
-
Tau-targeting therapies for Alzheimer disease: current status and future directions
Nature Reviews Neurology (2023)
-
Ultrasensitive and point-of-care detection of plasma phosphorylated tau in Alzheimer’s disease using colorimetric and surface-enhanced Raman scattering dual-readout lateral flow assay
Nano Research (2023)
-
Chronic Sustained Hypoxia Leads to Brainstem Tauopathy and Declines the Power of Rhythms in the Ventrolateral Medulla: Shedding Light on a Possible Mechanism
Molecular Neurobiology (2023)
-
Multidimensional insights into the repeated electromagnetic field stimulation and biosystems interaction in aging and age-related diseases
Journal of Biomedical Science (2022)