Abstract
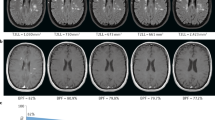
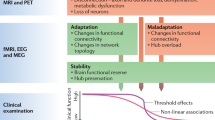
Multiple sclerosis (MS) is commonly regarded as an inflammatory disease, but it also has a neurodegenerative component, which represents an additional target for treatment. The use of MRI to evaluate the inflammatory disease component in 'proof-of concept' clinical trials is well established, but no systematic assessment of imaging outcomes to evaluate neuroprotection or repair in MS has been performed. In this Review, we examine the potential of traditional and novel imaging parameters to serve as primary outcomes in phase II clinical trials of neuroprotective and reparative strategies in MS. We present the conclusions of an international meeting of imaging, clinical and statistical experts, as well as a review of relevant literature. The available imaging techniques are appraised in five categories of performance: pathological specificity, reproducibility, sensitivity to change, clinical relevance, and response to treatment. At present, the three most promising primary outcomes in phase II trials of neuroprotective and/or reparative strategies in MS are: changes in whole-brain volume to gauge general cerebral atrophy; T1 hypointensity and magnetization transfer ratio to monitor the evolution of lesion damage; and optical coherence tomography findings to evaluate the anterior visual pathway. Power calculations show that these outcome measures can be applied with attainable sample sizes.
Key Points
-
Multiple sclerosis (MS) is not only an inflammatory demyelinating disease; it also involves neurodegeneration, which starts early in the disease process
-
Development of new neuroprotective and reparative treatments for MS has been hampered by lack of sensitive clinical response criteria
-
Imaging outcomes provide the best current in vivo measures of neuroprotection, and possibly also of repair, in MS
-
Brain-volume change on serial MRI provides a sensitive overall measure of neuroprotection in MS trials over the course of a year
-
Magnetization transfer ratio and T1 hypointensity provide lesional measures of neuroprotection and repair over the course of 6–9 months
-
Optical coherence tomography is a robust measure of axonal damage in the anterior visual pathways
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Compston, A. & Coles, A. Multiple sclerosis. Lancet 372, 1502–1517 (2008).
Lopez-Diego, R. S. & Weiner, H. L. Novel therapeutic strategies for multiple sclerosis—a multifaceted adversary. Nat. Rev. Drug Discov. 7, 909–925 (2008).
Miller, D. H. et al. Guidelines for the use of magnetic resonance techniques in monitoring the treatment of multiple sclerosis. US National MS Society Task Force. Ann. Neurol. 39, 6–16 (1996).
Edan, G. et al. Therapeutic effect of mitoxantrone combined with methylprednisolone in multiple sclerosis: a randomised multicentre study of active disease using MRI and clinical criteria. J. Neurol. Neurosurg. Psychiatry 62, 112–118 (1997).
Miller, D. H. et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 348, 68–72 (2003).
Polman, C. et al. Treatment with laquinimod reduces development of active MRI lesions in relapsing MS. Neurology 64, 987–991 (2005).
Kappos, L. et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N. Engl. J. Med. 255, 1124–1140 (2006).
O'Connor, P. W. et al. A phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology 66, 894–900 (2006).
Comi, G. et al. Effect of laquinimod on MRI-monitored disease activity in patients with relapsing–remitting multiple sclerosis: a multicentre, randomized, double-blind, placebo-controlled phase IIb study. Lancet 371, 2085–2092 (2008).
Kappos, L. et al. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 372, 1463–1472 (2008).
Zivadinov, R. et al. Mechanisms of action of disease-modifying agents and brain volume changes in multiple sclerosis. Neurology 71, 136–144 (2008).
Simon, J. H. Brain atrophy in multiple sclerosis: what we know and would like to know. Mult. Scler. 12, 679–687 (2006).
Anderson, V. M., Bartlett, J. W., Fox, N. C., Fisniku, L. & Miller, D. H. Detecting treatment effects on brain atrophy in relapsing remitting multiple sclerosis: sample size estimates. J. Neurol. 254, 1588–1594 (2007).
Altmann, D. R. et al. Sample sizes for brain atrophy outcomes in trials for secondary progressive multiple sclerosis. Neurology 72, 595–601 (2009).
Chen, J. T. et al. Brain atrophy after immunoablation and stem cell transplantation in multiple sclerosis. Neurology 66, 1935–1937 (2006).
Tiberio, M. et al. Gray and white matter volume changes in early RRMS: a 2-year longitudinal study. Neurology 64, 1001–1007 (2005).
Sastre-Garriga, J. et al. Metabolic changes in normal-appearing gray and white matter are linked with disability in early primary progressive multiple sclerosis. Arch. Neurol. 62, 569–573 (2005).
Fisher, E., Lee, J.-C., Nakamura, K. & Rudick, R. A. Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann. Neurol. 64, 255–265 (2008).
Nakamura, K. & Fisher, E. Segmentation of brain magnetic resonance images for measurement of gray matter atrophy in multiple sclerosis patients. Neuroimage 44, 769–776 (2009).
Barkhof, F. Assessing treatment effects on axonal loss – evidence from MRI monitored clinical trials. J. Neurol. 251 (Suppl. 4), IV6–IV12 (2004).
Bö, L., Geurts, J. J., Ravid, R. & Barkhof, F. Magnetic resonance imaging as a tool to examine the neuropathology of multiple sclerosis. Neuropath. Appl. Neurobiol. 30, 106–117 (2004).
Schmierer, K., Scaravilli, F., Altmann, D. R., Barker, G. J. & Miller, D. H. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann. Neurol. 56, 407–415 (2004).
Schmierer, K. et al. Quantitative magnetization transfer imaging in postmortem multiple sclerosis brain. J. Magn. Reson. Imaging 26, 41–51 (2007).
Giacomini, P. S. & Arnold, D. L. Non-conventional MRI techniques for measuring neuroprotection, repair and plasticity in multiple sclerosis. Curr. Opin. Neurol. 21, 272–277 (2008).
van den Elskamp, I. J. et al. Persistent T1 hypointensity as an MRI marker for treatment efficacy in multiple sclerosis. Mult. Scler. 14, 464–469 (2008).
MacKay, A. L. et al. MR relaxation in multiple sclerosis. Neuroimaging Clin. N. Am. 19, 1–26 (2009).
Bjartmar, C. & Trapp, B. D. Axonal degeneration and progressive neurological disability in multiple sclerosis. Neurotox. Res. 5, 157–164 (2003).
Ceccarelli, A. et al. Normal-appearing white and grey matter damage in MS. A volumetric and diffusion tensor MRI study at 3.0 Tesla. J. Neurol. 254, 513–518 (2007).
Davies, G. R. et al. Normal-appearing grey and white matter T1 abnormality in early relapsing–remitting multiple sclerosis: a longitudinal study. Mult. Scler. 13, 169–177 (2007).
Roosendaal, S. D. et al. Regional DTI differences in multiple sclerosis patients. Neuroimage 44, 1397–1403 (2009).
Reich, D. S. et al. Damage to the optic radiation in multiple sclerosis is associated with retinal injury and low contrast visual acuity. Arch. Neurol. in press (2009).
Miller, D. H., Thompson, A. J. & Filippi, M. Magnetic resonance studies of abnormalities in the normal appearing white matter and grey matter in multiple sclerosis. J. Neurol. 250, 1407–1419 (2003).
Rovaris, M. et al. Optimizing diffusion measurements for large-scale, multicenter multiple sclerosis trials: a pan-European study. Mult. Scler. 14 (Suppl.), S216 (2008).
Narayanan, S. et al. Axonal metabolic recovery in multiple sclerosis patients treated with interferon beta-1b. J. Neurol. 248, 979–986 (2001).
Khan, O. et al. Axonal metabolic recovery and potential neuroprotective effect of glatiramer acetate in relapsing-remitting multiple sclerosis. Mult. Scler. 11, 646–651 (2005).
Kutzelnigg, A. et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128, 2705–2712 (2005).
Stevenson, V. L. et al. Spinal cord atrophy and disability in MS: a longitudinal study. Neurology 51, 234–238 (1998).
Gilmore, C. et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult. Scler. 15, 180–188 (2009).
Bot, J. C. et al. Spinal cord abnormalities in recently diagnosed MS patients: added value of spinal MRI examination. Neurology 62, 226–233 (2004).
Jacobi, C. et al. Prospective combined brain and spinal cord MRI in clinically isolated syndromes and possible early multiple sclerosis: impact on dissemination in space and time. Eur. J. Neurol. 15, 1359–1364 (2008).
Agosta, F. et al. In vivo assessment of cervical cord damage in MS patients: a longitudinal diffusion tensor MRI study. Brain 130, 2211–2219 (2007).
Ciccarelli, O. et al. Spinal cord spectroscopy and diffusion-based tractography to assess acute disability in multiple sclerosis. Brain 130, 2220–2231 (2007).
DeBoy, C. A. et al. High resolution diffusion tensor imaging of axonal damage in focal inflammatory and demyelinating lesions in rat spinal cord. Brain 130, 2199–2210 (2007).
Budde, M. D. et al. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn. Reson. Med. 57, 688–695 (2007).
Sun, S. W., Liang, H. F., Cross, A. H. & Song, S. K. Evolving Wallerian degeneration after transient retinal ischemia in mice characterized by diffusion tensor imaging. Neuroimage 40, 1–10 (2008).
Sun, S. W., Liang, H. F., Xie, M., Oyoyo, U. & Lee, A. Fixation, not death, reduces sensitivity of DTI in detecting optic nerve damage. Neuroimage 44, 611–619 (2009).
Kalkers, N. F., Barkhof, F., Bergers, E., van Schijndel, R. & Polman, C. H. The effect of the neuroprotective agent riluzole on MRI parameters in primary progressive multiple sclerosis: a pilot study. Mult. Scler. 8, 532–533 (2002).
Balcer, L. J. et al. New low-contrast vision charts: reliability and test characteristics in patients with multiple sclerosis. Mult. Scler. 6, 163–171 (2000).
Baier, M. L. et al. Low-contrast letter acuity testing captures visual dysfunction in patients with multiple sclerosis. Neurology 64, 992–995 (2005).
Wu, G. F. et al. Relation of vision to global and regional brain MRI in multiple sclerosis. Neurology 69, 2128–2135 (2007).
Balcer, L. J. et al. Natalizumab reduces visual loss in patients with relapsing multiple sclerosis. Neurology 68, 1299–1304 (2007).
Trip, S. A. et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann. Neurol. 58, 383–391 (2005).
Fisher, J. B. et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology 113, 324–332 (2006).
Pulicken, M. et al. Optical coherence tomography and disease subtype in multiple sclerosis. Neurology 69, 2085–2092 (2007).
Gordon-Lipkin, E. et al. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology 69, 1603–1609 (2007).
Cettomai, D. et al. Reproducibility of optical coherence tomography in multiple sclerosis. Arch. Neurol. 65, 1218–1222 (2008).
Costello, F. et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann. Neurol. 59, 963–969 (2006).
Ciccarelli, O. et al. Optic radiation changes after optic neuritis detected by tractography-based group mapping. Hum. Brain Mapp. 25, 308–316 (2005).
Audoin, B. et al. Selective magnetization transfer ratio decrease in the visual cortex following optic neuritis. Brain 129, 1031–1039 (2006).
Hickman, S. J. et al. Optic nerve diffusion measurement from diffusion-weighted imaging in optic neuritis. Am. J. Neuroradiol. 26, 951–956 (2005).
Trip, S. A. et al. Optic nerve atrophy and retinal nerve fibre layer thinning following optic neuritis: evidence that axonal loss is a substrate of MRI-detected atrophy. Neuroimage 31, 286–293 (2006).
Trip, S. A. et al. Optic nerve magnetization transfer imaging and measures of axonal loss and demyelination in optic neuritis. Mult. Scler. 13, 875–879 (2007).
Wegner, C. et al. Relating functional changes during hand movement to clinical parameters in patients with multiple sclerosis in a multi-centre fMRI study. Eur. J. Neurol. 15, 113–122 (2008).
Bosnell, R. et al. Reproducibility of fMRI in the clinical setting: implications for trial designs. Neuroimage 42, 603–610 (2008).
Filippi, M. & Rocca, M. A. Application of fMRI to the study of multiple sclerosis: an update. Future Neurol. 3, 141–151 (2008).
Parry, A. M., Scott, R. B., Palace, J., Smith, S. & Matthews, P. M. Potentially adaptive functional changes in cognitive processing for patients with multiple sclerosis and their acute modulation by rivastigmine. Brain 126, 2750–2760 (2003).
Rocca, M. A. et al. fMRI changes in relapsing–remitting multiple sclerosis patients complaining of fatigue after IFNbeta-1a injection. Hum. Brain Mapp. 28, 373–382 (2007).
Xiang, Z. et al. Detection of myelination using a novel histological probe. J. Histochem. Cytochem. 53, 1511–1516 (2005).
Stankoff, B. et al. Imaging of CNS myelin by positron-emission tomography. Proc. Natl Acad. Sci. USA 103, 9304–9309 (2006).
Wu, C. et al. A novel fluorescent probe that is brain permeable and selectively binds to myelin. J. Histochem. Cytochem. 54, 997–1004 (2006).
Vowinckel, E. et al. PK11195 binding to the peripheral benzodiazepine receptor as a marker of microglia activation in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neurosci. Res. 50, 345–353 (1997).
Banati, R. B. et al. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain 123, 2321–2337 (2000).
Debruyne, J. C. et al. PET visualization of microglia in multiple sclerosis patients using [11C]PK11195. Eur. J. Neurol. 10, 257–264 (2003).
Rashid, W. et al. Abnormalities of cerebral perfusion in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 75, 1288–1293 (2004).
Adhya, S. et al. Pattern of hemodynamic impairment in multiple sclerosis: dynamic susceptibility contrast perfusion MR imaging at 3.0 T. Neuroimage 33, 1029–1035 (2006).
Wuerfel, J., Paul, F. & Zipp, F. Cerebral blood perfusion changes in multiple sclerosis. J. Neurol. Sci. 259, 16–20 (2007).
Inglese, M. et al. Perfusion magnetic resonance imaging correlates of neuropsychological impairment in multiple sclerosis. J. Cereb. Blood Flow Metab. 28, 164–171 (2008).
Ben-Hur, T. et al. Serial in vivo MR tracking of magnetically labeled neural spheres transplanted in chronic EAE mice. Magn. Reson. Med. 57, 164–171 (2007).
Zhu J., Zhou, L. & XingWu, F. Tracking neural stem cells in patients with brain trauma. N. Engl. J. Med. 355, 2376–2378 (2006).
Walczak, P. & Bulte, J. W. M. The role of noninvasive cellular imaging in developing cell-based therapies for neurodegenerative disorders. Neurodegenerative Dis. 4, 306–313 (2007).
Gilad, A. A. et al. Artificial reporter gene providing MRI contrast based on proton exchange. Nat. Biotechnol. 25, 217–219 (2007).
Vavasour, I. M. et al. Longitudinal changes in myelin water fraction in two MS patients with active disease. J. Neurol. Sci. 276, 49–53 (2009).
Acknowledgements
The workshop on Imaging Outcomes for Protection and Repair in Multiple Sclerosis in Amsterdam, The Netherlands (28–29 August 2008) was supported by the National Multiple Sclerosis Society (New York, USA) as an activity of its International Advisory Committee on Clinical Trials in Multiple Sclerosis. The Amsterdam MS Center is supported by the Dutch Foundation for MS Research (Voorschoten, The Netherlands). The Johns Hopkins Multiple Sclerosis Center is supported by the National MS Society (USA), the Nancy Davis Foundation and the National Institute of Neurological Disorders and Stroke, NIH (Bethesda, MD, USA). The NMR Research Unit at the Institute of Neurology, London, UK is supported by the Multiple Sclerosis Society of Great Britain and Northern Ireland. We thank Hanneke Hulst for providing Figure 2 and Paul Matthews for providing a template from which Figure 4 was adapted. We also thank Douglas Arnold and Laura Balcer for providing details about the power calculations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Box 1
Participant list for Imaging Outcomes for Protection and Repair in Multiple Sclerosis, 28–29 August 2008, Amsterdam, The Netherlands (DOC 32 kb)
Rights and permissions
About this article
Cite this article
Barkhof, F., Calabresi, P., Miller, D. et al. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol 5, 256–266 (2009). https://doi.org/10.1038/nrneurol.2009.41
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2009.41
This article is cited by
-
Don’t be late! Postponing cognitive decline and preventing early unemployment in people with multiple sclerosis: a study protocol
BMC Neurology (2024)
-
Evolution of acute “black hole” lesions in patients with relapsing–remitting multiple sclerosis
Acta Neurologica Belgica (2023)
-
Commercial volumetric MRI reporting tools in multiple sclerosis: a systematic review of the evidence
Neuroradiology (2023)
-
Quantitative MRI phenotypes capture biological heterogeneity in multiple sclerosis patients
Scientific Reports (2021)
-
A focus on secondary progressive multiple sclerosis (SPMS): challenges in diagnosis and definition
Journal of Neurology (2021)