Key Points
-
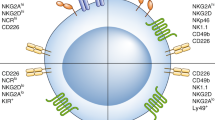
Natural killer (NK) cells have the ability to lyse tumour cells without the requirement for prior immune sensitization of the host. NK-cell recognition of target cells is tightly regulated by processes involving the integration of signals delivered from multiple activating and inhibitory receptors.
-
Insights into the molecular specificities that regulate NK-cell function have led to new possibilities to design NK-cell-based immunotherapeutic strategies against human cancer. Strategies of NK-cell immunotherapy include activation of endogenous NK cells, NK-cell-mediated graft-versus-tumour (GVT) effects in the context of haematopoietic allogeneic stem-cell transplantation (SCT), and adoptive transfer of allogeneic NK cells.
-
Endogenous NK cells may be activated by cytokines, immunomodulatory drugs, and agonists of activating receptors or by blockade of inhibitory killer-cell immunoglobulin-like receptor (KIR) with monoclonal antibodies, thereby augmenting tumour-cell recognition by NK cells. NK cells may also be genetically engineered to shift the balance towards NK-cell activation.
-
NK cells have been shown to mediate GVT effects in allogeneic haematopoietic SCT. Future criteria for donor selection may involve the selection of KIR–ligand-mismatched donors with a large alloreactive NK-cell subset.
-
Conditioning regimens will probably be required for survival and in vivo expansion of adoptively transferred NK cells. Apart from preventing rejection, such regimens may eradicate regulatory T cells and promote access to homeostatic cytokines, including IL-15.
-
Tumour-cell susceptibility to NK-cell lysis may be predicted by characterizing the expression of activating receptor ligands on tumour cells, as well as the expression of ligands for inhibitory receptors (MHC class I molecules). Such phenotypic analysis may be combined with direct testing of freshly isolated tumour cells for their susceptibility to NK-cell lysis ex vivo.
-
Combinatorial therapies, in which NK cells represent one important mediator, may further potentiate the clinical efficacy of NK-cell immunotherapy.
Abstract
Current insights into the molecular specificities that regulate natural killer (NK)-cell function suggest that it might be possible to design NK-cell-based immunotherapeutic strategies against human cancer. Here, we describe evidence for NK-cell targeting of human tumours and address crucial questions that, in our opinion, require consideration for the development of successful NK-cell-based therapies. Appropriately used, we predict that NK cells will have a role, both directly and in combination with other treatment modalities, in future treatment of cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kiessling, R., Klein, E. & Wigzell, H. 'Natural' killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur. J. Immunol. 5, 112–117 (1975).
Herberman, R. B., Nunn, M. E. & Lavrin, D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int. J. Cancer. 16, 216–229 (1975). The authors of references 1 and 2 independently report their parallel discovery of NK cells. These cells were named 'natural' killer cells by Kiessling and collaborators in reference 1.
Colucci, F., Caligiuri, M. A. & Di Santo, J. P. What does it take to make a natural killer? Nature Rev. Immunol. 3, 413–425 (2003).
Raulet, D. H. Interplay of natural killer cells and their receptors with the adaptive immune response. Nature Immunol. 5, 996–1002 (2004).
Lanier, L. L. NK cell recognition. Annu. Rev. Immunol. 23, 225–274 (2005).
Farag, S. S. & Caligiuri, M. A. Human natural killer cell development and biology. Blood Rev. 20, 123–137 (2006).
Biron, C. A., Nguyen, K. B., Pien, G. C., Cousens, L. P. & Salazar-Mather, T. P. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17, 189–220 (1999).
Wu, J. & Lanier, L. L. Natural killer cells and cancer. Adv. Cancer Res. 90, 127–156 (2003).
Cooper, M. A., Fehniger, T. A. & Caligiuri, M. A. The biology of human natural killer-cell subsets. Trends Immunol. 22, 633–640 (2001).
Moretta, L. & Moretta, A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 23, 255–259 (2004).
Ljunggren, H. G. & Kärre, K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J. Exp. Med. 162, 1745–1759 (1985).
Kärre, K., Ljunggren, H. G., Piontek, G. & Kiessling, R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 319, 675–678 (1986). References 11 and 12 describe the NK-cell dependent rejection of syngeneic tumour cells that lack expression of self MHC class I molecules.
Ljunggren, H. G. & Kärre, K. In search of the 'missing self': MHC molecules and NK cell recognition. Immunol. Today 11, 237–244 (1990).
Moretta, A. et al. A novel surface antigen expressed by a subset of human CD3− CD16+ natural killer cells. Role in cell activation and regulation of cytolytic function. J. Exp. Med. 171, 695–714 (1990).
Moretta, A. et al. Identification of four subsets of human CD3−CD16+ natural killer (NK) cells by the expression of clonally distributed functional surface molecules: correlation between subset assignment of NK clones and ability to mediate specific alloantigen recognition. J. Exp. Med. 172, 1589–1598 (1990). References 14 and 15 describe monoclonal antibodies to NK-cell subsets, paving the way for the identification of human NK-cell inhibitory receptors recognizing MHC class I.
Moretta, A. et al. P58 molecules as putative receptors for major histocompatibility complex (MHC) class I molecules in human natural killer (NK) cells. Anti-p58 antibodies reconstitute lysis of MHC class I-protected cells in NK clones displaying different specificities. J. Exp. Med. 178, 597–604 (1993). This study shows that P58-specific antibodies can disrupt the NK-cell mediated recognition of HLA class I molecules, thereby providing the first molecular identification of HLA class I-specific inhibitory receptors
Braud, V. M. et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 391, 795–799 (1998).
Vitale, M. et al. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J. Exp. Med. 187, 2065–2072 (1998).
Pessino, A. et al. Molecular cloning of NKp46: a novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J. Exp. Med. 188, 953–960 (1998). References 18 and 19 describe the identification of the natural cytotoxicity receptors NKp44 and NKp46.
Bauer, S. et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285, 727–729 (1999). This paper identifies NKG2D as a receptor for the stress-induced molecule MICA.
Bryceson, Y. T., March, M. E., Ljunggren, H. G. & Long, E. O. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol. Rev. 214, 73–91 (2006).
Hayakawa, Y. & Smyth, M. J. Innate immune recognition and suppression of tumors. Adv. Cancer Res. 95, 293–322 (2006).
Fehniger, T. A., Cooper, M. A. & Caligiuri, M. A. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Factor Rev. 13, 169–183 (2002).
Ruggeri, L. et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295, 2097–2100 (2002). This is a seminal paper describing that donor-versus-recipient NK-cell alloreactivity can eliminate leukaemia relapse and graft rejection, and protect against GVHD in human SCT.
Miller, J. S. et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 105, 3051–3057 (2005). This is a key study in which Miller and collaborators test haploidentical NK-cell infusions in a non-transplantation setting, to determine safety and in vivo NK-cell expansion.
Ruggeri, L., Aversa, F., Martelli, M. F. & Velardi, A. Allogeneic hematopoietic transplantation and natural killer cell recognition of missing self. Immunol. Rev. 214, 202–218 (2006).
Farag, S. S. & Caligiuri, M. A. Cytokine modulation of the innate immune system in the treatment of leukemia and lymphoma. Adv. Pharmacol. 51, 295–318 (2004).
Smyth, M. J., Cretney, E., Kershaw, M. H. & Hayakawa, Y. Cytokines in cancer immunity and immunotherapy. Immunol. Rev. 202, 275–293 (2004).
Becknell, B. & Caligiuri, M. A. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv. Immunol. 86, 209–239 (2005).
Rosenberg, S. A. Interleukin-2 and the development of immunotherapy for the treatment of patients with cancer. Cancer J. Sci. Am. 6 (Suppl. 1), 2–7 (2000).
Colombo, M. P. & Trinchieri, G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 13, 155–168 (2002).
Hayashi, T. et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical application. Br. J. Haematol. 128, 192–203 (2005).
Brandau, S. et al. NK cells are essential for effective BCG immunotherapy. Int. J. Cancer 92, 697–702 (2001).
Link, B. K. et al. Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin lymphoma. J. Immunother. 29, 558–568 (2006).
Fujii, H. et al. In vivo control of acute lymphoblastic leukemia by immunostimulatory CpG oligonucleotides. Blood 109, 2008–2013 (2007).
Ruggeri, L. et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood 94, 333–339 (1999).
Shlomchik, W. D. et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science 285, 412–415 (1999).
Giebel, S. et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood 102, 814–819 (2003).
Beelen, D. W. et al. Genotypic inhibitory killer immunoglobulin-like receptor ligand incompatibility enhances the long-term antileukemic effect of unmodified allogeneic hematopoietic stem cell transplantation in patients with myeloid leukemias. Blood 105, 2594–2600 (2005).
Miller, J. S. et al. Missing KIR-ligands is associated with less relapse and increased graft versus host disease (GVHD) following unrelated donor allogeneic HCT. Blood (28 March 2007 (doi:10.1182/blood-2006-07-036228).
Davies, S. M. et al. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Killer immunoglobulin-like receptor. Blood 100, 3825–3827 (2002).
Bornhauser, M. et al. Role of KIR ligand incompatibility in hematopoietic stem cell transplantation using unrelated donors. Blood. 103, 2860–2861 (2004).
Schaffer, M., Malmberg, K. J., Ringden, O., Ljunggren, H. G. & Remberger, M. Increased infection-related mortality in KIR-ligand-mismatched unrelated allogeneic hematopoietic stem-cell transplantation. Transplantation 78, 1081–1085 (2004).
Farag, S. S. et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol. Blood Marrow Transplant. 12, 876–884 (2006).
Aversa, F. et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N. Engl. J. Med. 339, 1186–1193 (1998).
Shilling, H. G. et al. Reconstitution of NK cell receptor repertoire following HLA-matched hematopoietic cell transplantation. Blood 101, 3730–3740 (2003).
Cooley, S. et al. KIR reconstitution is altered by T cells in the graft and correlates with clinical outcomes after unrelated donor transplantation. Blood 106, 4370–4376 (2005).
Guma, M. et al. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood 104, 3664–3671 (2004).
Fernandez, N. C. et al. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood 105, 4416–4423 (2005).
Kim, S. et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature 436, 709–713 (2005).
Anfossi, N. et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity 25, 331–342 (2006).
Yokoyama, W. M. & Kim, S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol. Rev. 214, 143–154 (2006).
Gasser, S. & Raulet, D. H. Activation and self-tolerance of natural killer cells. Immunol. Rev. 214, 130–142 (2006).
Leung, W. et al. Determinants of antileukemia effects of allogeneic NK cells. J. Immunol. 172, 644–650 (2004).
Hsu, K. C. et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood 105, 4878–4884 (2005).
Kolb, H. J. et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood 76, 2462–2465 (1990). This is a study that describes the first transfusion of viable donor lymphocytes to three patients with haematological relapse following SCT.
Kolb, H. J., Simoes, B. & Schmid, C. Cellular immunotherapy after allogeneic stem cell transplantation in hematologic malignancies. Curr. Opin. Oncol. 16, 167–173 (2004).
Soiffer, R. J. et al. Randomized trial of CD8+ T-cell depletion in the prevention of graft-versus-host disease associated with donor lymphocyte infusion. Biol. Blood Marrow Transplant. 8, 625–632 (2002).
Passweg, J. R. et al. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia 18, 1835–1838 (2004).
Passweg, J. R., Stern, M., Koehl, U., Uharek, L. & Tichelli, A. Use of natural killer cells in hematopoetic stem cell transplantation. Bone Marrow Transplant. 35, 637–643 (2005).
Bottino, C., Castriconi, R., Moretta, L. & Moretta, A. Cellular ligands of activating NK receptors. Trends Immunol. 26, 221–226 (2005).
Gonzalez, S., Groh, V. & Spies, T. Immunobiology of human NKG2D and its ligands. Curr. Top. Microbiol. Immunol. 298, 121–138 (2006).
Lundqvist, A., McCoy, J. P., Samsel, L. & Childs, R. Reduction of GVHD and enhanced anti-tumor effects after adoptive infusion of alloreactive Ly49-mismatched NK-cells from MHC-matched donors. Blood 109, 3603?3606 (2006).
Rosenberg, S. A. et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N. Engl. J. Med. 313, 1485–1492 (1985). This is a pioneering study that describes systemic administration of autologous lymphokine activated killer cells and IL-2 to patients with advanced cancer. This study stimulated the development of new protocols for adoptive-cell-mediated immunotherapy.
Law, T. M. et al. Phase III randomized trial of interleukin-2 with or without lymphokine-activated killer cells in the treatment of patients with advanced renal cell carcinoma. Cancer 76, 824–832 (1995).
Dudley, M. E. et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298, 850–854 (2002). This is a recent study that suggests the therapeutic potential of genetically engineered T cells for immunotherapy of cancer.
Gattinoni, L., Powell, D. J., Jr., Rosenberg, S. A. & Restifo, N. P. Adoptive immunotherapy for cancer: building on success. Nature Rev. Immunol. 6, 383–393 (2006). This is an excellent review that discusses prospects for T-cell-mediated immunotherapy, in particular in relation to strategies for host preconditioning by lymphodepletion.
Burns, L. J. et al. IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: a phase I/II trial. Bone Marrow Transplant. 32, 177–186 (2003).
Tam, Y. K., Martinson, J. A., Doligosa, K. & Klingemann, H. G. Ex vivo expansion of the highly cytotoxic human natural killer-92 cell-line under current good manufacturing practice conditions for clinical adoptive cellular immunotherapy. Cytotherapy 5, 259–272 (2003).
Klingemann, H. G. Natural killer cell-based immuno-therapeutic strategies. Cytotherapy 7, 16–22 (2005).
Carlens, S. et al. A new method for in vitro expansion of cytotoxic human CD3−CD56+ natural killer cells. Hum. Immunol. 62, 1092–1098 (2001).
Klingemann, H. G. & Martinson, J. Ex vivo expansion of natural killer cells for clinical applications. Cytotherapy 6, 15–22 (2004).
Gattinoni, L. et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J. Clin. Invest. 115, 1616–1626 (2005).
Ruggeri, L., Mancusi, A., Capanni, M., Martelli, M. F. & Velardi, A. Exploitation of alloreactive NK cells in adoptive immunotherapy of cancer. Curr. Opin. Immunol. 17, 211–217 (2005).
Pende, D. et al. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112). Blood 105, 2066–2073 (2005).
Stewart, C. A. et al. Recognition of peptide-MHC class I complexes by activating killer immunoglobulin-like receptors. Proc. Natl Acad. Sci. USA 102, 13224–13229 (2005).
Ghiringhelli, F. et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-b-dependent manner. J. Exp. Med. 202, 1075–1085 (2005).
Muranski, P. et al. Increased intensity lymphodepletion and adoptive immunotherapy—how far can we go? Nature Clin. Pract. Oncol. 3, 668–681 (2006).
Sheridan, C. First-in-class cancer therapeutic to stimulate natural killer cells. Nature Biotechnol. 24, 597 (2006).
Koh, C. Y. et al. Augmentation of antitumor effects by NK cell inhibitory receptor blockade in vitro and in vivo. Blood 97, 3132–3137 (2001).
Koh, C. Y., Ortaldo, J. R., Blazar, B. R., Bennett, M. & Murphy, W. J. NK-cell purging of leukemia: superior antitumor effects of NK cells H2 allogeneic to the tumor and augmentation with inhibitory receptor blockade. Blood 102, 4067–4075 (2003).
Zhang, T., Barber, A. & Sentman, C. L. Generation of antitumor responses by genetic modification of primary human T cells with a chimeric NKG2D receptor. Cancer Res. 66, 5927–5933 (2006).
Imai, C., Iwamoto, S. & Campana, D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 106, 376–383 (2005).
Uherek, C. et al. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood 100, 1265–1273 (2002).
Bryceson, Y. T., March, M. E., Ljunggren, H. G. & Long, E. O. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood 107, 159–166 (2006).
Castriconi, R. et al. Natural killer cell-mediated killing of freshly isolated neuroblastoma cells: critical role of DNAX accessory molecule-1-poliovirus receptor interaction. Cancer Res. 64, 9180–9184 (2004).
Moretta, L. et al. Surface NK receptors and their ligands on tumor cells. Semin. Immunol. 18, 151–158 (2006).
Carlsten, M. et al. DNAX accessory molecule-1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells. Cancer Res. 67, 1317–1325 (2007).
Malmberg, K. J. & Ljunggren, H. G. Escape from immune- and nonimmune-mediated tumor surveillance. Semin. Cancer Biol. 16, 16–31 (2006).
Albertsson, P. A. et al. NK cells and the tumour microenvironment: implications for NK-cell function and anti-tumour activity. Trends Immunol. 24, 603–609 (2003).
Morris, M. A. & Ley, K. Trafficking of natural killer cells. Curr. Mol. Med. 4, 431–438 (2004).
Ahrens, E. T., Flores, R., Xu, H. & Morel, P. A. In vivo imaging platform for tracking immunotherapeutic cells. Nature Biotechnol. 23, 983–987 (2005).
Castriconi, R. et al. Transforming growth factor β1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc. Natl Acad. Sci. USA 100, 4120–4125 (2003).
Chiesa, M. D. et al. The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood 108, 4118–4125 (2006).
Gasser, S., Orsulic, S., Brown, E. J. & Raulet, D. H. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 436, 1186–1190 (2005). This paper describes how DNA damage can alert the immune system to the presence of potentially dangerous cells.
Gasser, S. & Raulet, D. The DNA damage response, immunity and cancer. Semin. Cancer Biol. 16, 344–347 (2006).
Sayers, T. J. et al. The proteasome inhibitor PS-341 sensitizes neoplastic cells to TRAIL-mediated apoptosis by reducing levels of c-FLIP. Blood 102, 303–310 (2003).
VanOosten, R. L., Moore, J. M., Karacay, B. & Griffith, T. S. Histone deacetylase inhibitors modulate renal cell carcinoma sensitivity to TRAIL/Apo-2L-induced apoptosis by enhancing TRAIL-R2 expression. Cancer Biol. Ther. 4, 1104–1112 (2005).
Lundqvist, A. et al. Bortezomib and depsipeptide sensitize tumors to tumor necrosis factor-related apoptosis-inducing ligand: a novel method to potentiate natural killer cell tumor cytotoxicity. Cancer Res. 66, 7317–7325 (2006).
Skov, S. et al. Cancer cells become susceptible to natural killer cell killing after exposure to histone deacetylase inhibitors due to glycogen synthase kinase-3-dependent expression of MHC class I-related chain A and B. Cancer Res. 65, 11136–11145 (2005).
Reff, M. E. et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 83, 435–445 (1994).
Weng, W. K. & Levy, R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 21, 3940–3947 (2003).
Dall'Ozzo, S. et al. Rituximab-dependent cytotoxicity by natural killer cells: influence of FCGR3A polymorphism on the concentration-effect relationship. Cancer Res. 64, 4664–4669 (2004).
Adams, G. P. & Weiner, L. M. Monoclonal antibody therapy of cancer. Nature Biotechnol. 23, 1147–1157 (2005).
Carter, P. J. Potent antibody therapeutics by design. Nature Rev. Immunol. 6, 343–357 (2006).
Gluck, W. L. et al. Phase I studies of interleukin (IL)-2 and rituximab in B-cell non-hodgkin's lymphoma: IL-2 mediated natural killer cell expansion correlations with clinical response. Clin. Cancer Res. 10, 2253–2264 (2004).
Curti, B. D. Immunomodulatory and antitumor effects of interleukin-21 in patients with renal cell carcinoma. Expert Rev. Anticancer Ther. 6, 905–909 (2006).
Hartmann, F. et al. Anti-CD16/CD30 bispecific antibody treatment for Hodgkin's disease: role of infusion schedule and costimulation with cytokines. Clin. Cancer Res. 7, 1873–1881 (2001).
Shahied, L. S. et al. Bispecific minibodies targeting HER2/neu and CD16 exhibit improved tumor lysis when placed in a divalent tumor antigen binding format. J. Biol. Chem. 279, 53907–53914 (2004).
Bruenke, J. et al. Effective lysis of lymphoma cells with a stabilised bispecific single-chain Fv antibody against CD19 and FcγRIII (CD16). Br. J. Haematol. 130, 218–228 (2005).
Cosman, D. et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity 14, 123–133 (2001).
Pende, D. et al. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J. Exp. Med. 190, 1505–1516 (1999).
Vitale, M. et al. Identification of NKp80, a novel triggering molecule expressed by human NK cells. Eur. J. Immunol. 31, 233–242 (2001).
Welte, S., Kuttruff, S., Waldhauer, I. & Steinle, A. Mutual activation of natural killer cells and monocytes mediated by NKp80–AICL interaction. Nature Immunol. 7, 1334–1342 (2006).
Bottino, C. et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J. Exp. Med. 198, 557–567 (2003).
Fuchs, A., Cella, M., Giurisato, E., Shaw, A. S. & Colonna, M. Cutting edge: CD96 (tactile) promotes NK cell-target cell adhesion by interacting with the poliovirus receptor (CD155). J. Immunol. 172, 3994–3998 (2004).
Anegon, I., Cuturi, M. C., Trinchieri, G. & Perussia, B. Interaction of Fc receptor (CD16) ligands induces transcription of interleukin 2 receptor (CD25) and lymphokine genes and expression of their products in human natural killer cells. J. Exp. Med. 167, 452–472 (1988).
Biassoni, R. et al. Role of amino acid position 70 in the binding affinity of p50.1 and p58.1 receptors for HLA-Cw4 molecules. Eur. J. Immunol. 27, 3095–3099 (1997).
Vales-Gomez, M., Reyburn, H. T., Erskine, R. A. & Strominger, J. Differential binding to HLA-C of p50-activating and p58-inhibitory natural killer cell receptors. Proc. Natl Acad. Sci. USA 95, 14326–14331 (1998).
O'Connor, G. M. et al. Functional polymorphism of the KIR3DL1/S1 receptor on human NK cells. J. Immunol. 178, 235–241 (2007).
Carr, W. H. et al. Cutting Edge: KIR3DS1, a gene implicated in resistance to progression to AIDS, encodes a DAP12-associated receptor expressed on NK cells that triggers NK cell activation. J. Immunol. 178, 647–651 (2007).
Le Bouteiller, P. et al. Engagement of CD160 receptor by HLA-C is a triggering mechanism used by circulating natural killer (NK) cells to mediate cytotoxicity. Proc. Natl Acad. Sci. USA 99, 16963–16968 (2002).
Selvaraj, P. et al. The T lymphocyte glycoprotein CD2 binds the cell surface ligand LFA-3. Nature 326, 400–403 (1987).
Winter, C. C., Gumperz, J. E., Parham, P., Long, E. O. & Wagtmann, N. Direct binding and functional transfer of NK cell inhibitory receptors reveal novel patterns of HLA-C allotype recognition. J. Immunol. 161, 571–577 (1998).
Colonna, M. et al. Alloantigen recognition by two human natural killer cell clones is associated with HLA-C or a closely linked gene. Proc. Natl Acad. Sci. USA 89, 7983–7985 (1992).
Cella, M., Longo, A., Ferrara, G. B., Strominger, J. L. & Colonna, M. NK3-specific natural killer cells are selectively inhibited by Bw4-positive HLA alleles with isoleucine 80. J. Exp. Med. 180, 1235–1242 (1994).
Pende, D. et al. The natural killer cell receptor specific for HLA-A allotypes: a novel member of the p58/p70 family of inhibitory receptors that is characterized by three immunoglobulin-like domains and is expressed as a 140-kD disulphide-linked dimer. J. Exp. Med. 184, 505–518 (1996).
Dohring, C., Scheidegger, D., Samaridis, J., Cella, M. & Colonna, M. A human killer inhibitory receptor specific for HLA-A1,2. J. Immunol. 156, 3098–3101 (1996).
Hansasuta, P. et al. Recognition of HLA-A3 and HLA-A11 by KIR3DL2 is peptide-specific. Eur. J. Immunol. 34, 1673–1679 (2004).
Cosman, D. et al. A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity 7, 273–282 (1997).
Colonna, M. et al. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J. Exp. Med. 186, 1809–1818 (1997).
Butcher, S., Arney, K. L. & Cook, G. P. MAFA-L, an ITIM-containing receptor encoded by the human NK cell gene complex and expressed by basophils and NK cells. Eur. J. Immunol. 28, 3755–3762 (1998).
Ito, M. et al. Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit NK cell cytotoxicity. J. Exp. Med. 203, 289–295 (2006).
Rosen, D. B. et al. Cutting edge: lectin-like transcript-1 is a ligand for the inhibitory human NKR-P1A receptor. J. Immunol. 175, 7796–7799 (2005).
Aldemir, H. et al. Cutting edge: lectin-like transcript 1 is a ligand for the CD161 receptor. J. Immunol. 175, 7791–7795 (2005).
Nicoll, G. et al. Identification and characterization of a novel siglec, siglec-7, expressed by human natural killer cells and monocytes. J. Biol. Chem. 274, 34089–34095 (1999).
Brown, M. H. et al. 2B4, the natural killer and T cell immunoglobulin superfamily surface protein, is a ligand for CD48. J. Exp. Med. 188, 2083–2090 (1998).
Latchman, Y., McKay, P. F. & Reiser, H. Identification of the 2B4 molecule as a counter-receptor for CD48. J. Immunol. 161, 5809–5812 (1998).
Bottino, C. et al. NTB-A, a novel SH2D1A-associated surface molecule contributing to the inability of natural killer cells to kill Epstein–Barr virus-infected B cells in X-linked lymphoproliferative disease. J. Exp. Med. 194, 235–246 (2001).
Flaig, R. M., Stark, S. & Watzl, C. Cutting edge: NTB-A activates NK cells via homophilic interaction. J. Immunol. 172, 6524–6527 (2004).
Rajagopalan, S. & Long, E. O. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J. Exp. Med. 189, 1093–1100 (1999).
Acknowledgements
We thank our colleagues S. Applequist, B. Baumann, N. Björkström, Y. Bryceson, M. Carlsten, C. Fauriat, and P. Ljungman for critically reading the manuscript, as well as other members of our laboratory for fruitful discussions. We are supported by the Swedish Foundation for Strategic Research, the Swedish Research Council, the Swedish Cancer Society, the Tobias Foundation, the Swedish Children's Cancer Foundation, the Cancer Society of Stockholm, the Karolinska Institutet and the Karolinska University Hospital. K.J.M. is a research fellow at the Royal Swedish Academy of Sciences.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Antibody-dependent cell-mediated cytotoxicity
-
(ADCC). A mechanism by which natural killer (NK) cells are targeted to IgG-coated cells, resulting in the lysis of the antibody-coated cells. The low-affinity Fc receptor for IgG (FcγRIII; also known as CD16), is expressed at the surface of NK cells and mediates ADCC.
- Thalidomide and Lenalidomide
-
Thalidomide (α-N-phthalimidoglutarimide) is a derivative of glutamic acid. It is pharmacologically classified as an immunomodulatory drug because of its ability to change the expression of various cytokines and to co-stimulate immune effector cells including natural killer cells. Lenalidomide is a 4-aminoglutaramide analogue of thalidomide with enhanced immunomodulatory effects and a favourable toxicity profile compared with its parent compound.
- Immunostimulatory DNA complexes
-
Synthetic DNA oligonucleotides containing unmethylated CpG motifs (CpG-ODN) that provide a danger signal to the immune system by mimicking the activity of bacterial DNA. CpG-ODN trigger rapid responses by innate immune cells including plasmacytoid dendritic cells and natural killer cells.
- Licensing
-
A functional maturation process of NK cells involving the recognition of host MHC class I molecules by inhibitory NK-cell receptors. Lack of such functional maturation has been suggested as one mechanism behind the hyporesponsiveness of NK cells that do not express inhibitory receptors to host MHC class I molecules.
- Replicative senescence
-
A growth-arrest state eventually reached by cells that have undergone repetitive proliferation in vitro or in vivo, which is characterized by functionally active cells that lack, or have a reduced, proliferative capacity. Senescent immune cells are more common in elderly people and in patients with chronic inflammatory diseases.
- Regulatory T cells
-
(TReg cells). A small population of CD4+ T cells that expresses the transcription factor forkhead box P3 (FOXP3) and has regulatory (that is, suppressor) activity towards T-cell and natural-killer-cell activation. An absence of functional TReg-cells is associated with severe autoimmunity.
- Cyclophosphamide
-
A DNA-alkylating agent that is used widely as an antitumour agent or an immunosuppressive agent. Cyclophosphamide has been shown to destroy certain subsets of lymphocytes preferentially, including B cells and regulatory cells.
- Fludarabine
-
A purine analogue that acts as an inhibitory substrate for ribonucleotide reductase, DNA polymerases, DNA ligase I, and DNA primase. It affects DNA synthesis and transcription. Fludarabine induces cell death, particularly in leukaemic cells, and is commonly used in the treatment of indolent leukaemia and lymphoma. Fludarabine has also been incorporated into reduced intensity preparative regimens for haematopoietic stem-cell transplantation.
- Small interfering RNA
-
(siRNA). Short double-stranded RNAs of 19–23 nucleotides that induce RNA interference, a post-transcriptional process that leads to gene silencing in a sequence-specific manner.
- Bispecific antibodies
-
These are antibodies that are derived from the recombination of variable domains from two antibodies with different specificities. Bispecific antibodies have been used to redirect cytotoxic immune cells to tumour cells.
Rights and permissions
About this article
Cite this article
Ljunggren, HG., Malmberg, KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol 7, 329–339 (2007). https://doi.org/10.1038/nri2073
Issue Date:
DOI: https://doi.org/10.1038/nri2073
This article is cited by
-
Radiotherapy combined with nano-biomaterials for cancer radio-immunotherapy
Journal of Nanobiotechnology (2023)
-
Phase I non-randomized clinical trial of allogeneic natural killer cells infusion in acute myeloid leukemia patients
BMC Cancer (2023)
-
Peripheral blood lymphocyte subsets in children with nephrotic syndrome: a retrospective analysis
BMC Nephrology (2023)
-
Natural killer cell-derived exosomes for cancer immunotherapy: innovative therapeutics art
Cancer Cell International (2023)
-
The apoptotic effects of NK-92 cells stimulated with an anti-CD226 antibody on MDA-MB-231 triple-negative breast cancer cells
Medical Oncology (2023)