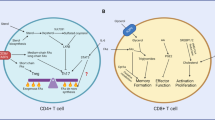
Key Points
-
For all cells, a constant supply of intracellular metabolites is required to sustain the most vital tasks of the cell. Nutrients such as glucose, amino acids and fatty acids can be degraded into simpler intermediates to provide metabolic energy in the form of ATP. The same intermediates can be used to build macromolecules such as proteins and lipids at the expense of ATP.
-
In the peripheral circulation, glucose, amino acids and fatty acids are maintained at relatively constant concentrations. In the absence of instructional extracellular signals that are delivered through the ligation of cytokine, antigen or co-stimulatory receptors, lymphocytes lack the ability to take up sufficient nutrients to maintain even their basic bioenergetic needs.
-
T cells have evolved the capacity to switch between states of relative quiescence and rapid proliferative expansion. These two fates are regulated, in part, by signals that are delivered through cytokine and antigen receptors, the outcome of which is closely coupled to the differentiation state of the responding cell.
-
Rather than a default response to a lack of mitogenic signals, quiescence in T cells is an actively maintained state with unique metabolic demands. Resting T cells derive most of their ATP from the oxidative phosphorylation of intracellular metabolites, and they use this energy to suppress actively the expression of cell-cycle proteins through regulated protein degradation.
-
Although activated lymphocytes are preparing to commit to the energy-demanding process of proliferation, they hyperinduce glycolysis and preferentially excrete pyruvate as lactate, even when oxygen is not present in limiting concentrations. This indicates that glucose uptake and utilization is a required part of the metabolic response of T cells to mitogenic signals.
-
Rapamycin, which inhibits TOR (target of rapamycin), is a powerful immunosuppressant that functions by targeting cellular metabolism. In addition to enzymes of the phosphatidylinositol-3-kinase pathway, the kinases PIM1 and PIM2 are important contributors to the rapamycin sensitivity of lymphocytes in vivo and in vitro.
Abstract
Ligation of antigen receptors at the surface of lymphocytes initiates a transcriptional and translational response that is required for cellular proliferation and effector function. By contrast, co-stimulatory-molecule ligation contributes to the immune response by allowing the uptake and utilization of extracellular nutrients to provide energy for cellular proliferation and effector functions. Growth factors also potentiate the ability of lymphocytes to metabolically switch between resting and proliferative states. Lymphocytes that do not receive these signals fail to increase their metabolism to meet the higher bioenergetic demands of cell growth and are either deleted or rendered unresponsive to mitogenic signals. In this Review, we describe how T cells actively acquire metabolic substrates from their environment to meet these energy demands and respond appropriately to pathogens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Singer, A. & Bosselut, R. CD4/CD8 coreceptors in thymocyte development, selection, and lineage commitment: analysis of the CD4/CD8 lineage decision. Adv. Immunol. 83, 91–131 (2004).
Bradley, L. M., Haynes, L. & Swain, S. L. IL-7: maintaining T-cell memory and achieving homeostasis. Trends Immunol. 26, 172–176 (2005).
Benczik, M. & Gaffen, S. L. The interleukin (IL)-2 family cytokines: survival and proliferation signaling pathways in T lymphocytes. Immunol. Invest. 33, 109–142 (2004).
Hammerman, P. S., Fox, C. J. & Thompson, C. B. Beginnings of a signal-transduction pathway for bioenergetic control of cell survival. Trends. Biochem. Sci. 29, 586–592 (2004).
Plas, D. R., Rathmell, J. C. & Thompson, C. B. Homeostatic control of lymphocyte survival: potential origins and implications. Nature Immunol. 3, 515–521 (2002).
Plas, D. R. & Thompson, C. B. Cell metabolism in the regulation of programmed cell death. Trends Endocrinol. Metab. 13, 75–78 (2002).
Cory, S. & Adams, J. M. The Bcl2 family: regulators of the cellular life-or-death switch. Nature Rev. Cancer 2, 647–656 (2002).
Lum, J. J. et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 120, 237–248 (2005).
Sbarra, A. J. & Karnovsky, M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J. Biol. Chem. 234, 1355–1362 (1959).
Borregaard, N. & Herlin, T. Energy metabolism of human neutrophils during phagocytosis. J. Clin. Invest. 70, 550–557 (1982).
Borregaard, N. & Kragballe, K. The oxygen-dependent antibody-dependent cell-mediated cytotoxicity of human monocytes and neutrophils. Adv. Exp. Med. Biol. 141, 71–84 (1982).
Cramer, T. et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112, 645–657 (2003). Using a gene-knockout-mouse approach, this paper shows that the oxygen sensor hypoxia-inducible factor 1α (HIF1α) is an essential regulator of the glycolytic capacity of myeloid cells in the anaerobic microenvironment of inflamed tissue. In the absence of HIF1α, the cellular ATP pool is reduced in activated monocytes, leading to their functional impairment.
Bauer, D. E. et al. Cytokine stimulation of aerobic glycolysis in hematopoietic cells exceeds proliferative demand. FASEB J. 18, 1303–1305 (2004).
Elstrom, R. L. et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 64, 3892–3899 (2004).
Krauss, S., Brand, M. D. & Buttgereit, F. Signaling takes a breath — new quantitative perspectives on bioenergetics and signal transduction. Immunity 15, 497–502 (2001).
Brookes, P. S., Yoon, Y., Robotham, J. L., Anders, M. W. & Sheu, S. S. Calcium, ATP, and ROS: a mitochondrial love–hate triangle. Am. J. Physiol. Cell Physiol. 287, C817–C833 (2004).
Frauwirth, K. A. & Thompson, C. B. Regulation of T lymphocyte metabolism. J. Immunol. 172, 4661–4665 (2004).
Rathmell, J. C., Vander Heiden, M. G., Harris, M. H., Frauwirth, K. A. & Thompson, C. B. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol. Cell 6, 683–692 (2000).
Buttgereit, F., Burmester, G. R. & Brand, M. D. Bioenergetics of immune functions: fundamental and therapeutic aspects. Immunol. Today 21, 192–199 (2000). In a series of papers (references 19, 24 and 25), the research group of Martin Brand uses quiescent rat thymocytes or thymocytes activated by mitogens such as concanavalin A in an attempt to trace the major oxygen- and/or ATP-consuming processes, including protein synthesis and cation transport.
Gray, J. V. et al. 'Sleeping beauty': quiescence in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 68, 187–206 (2004). This Review is highly recommended. It summarizes much of the literature concerning the regulated entry to, and exit from, quiescence in yeast.
Greiner, E. F., Guppy, M. & Brand, K. Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. J. Biol. Chem. 269, 31484–31490 (1994).
Brand, K. Glutamine and glucose metabolism during thymocyte proliferation. Pathways of glutamine and glutamate metabolism. Biochem. J. 228, 353–361 (1985).
Brand, K., Williams, J. F. & Weidemann, M. J. Glucose and glutamine metabolism in rat thymocytes. Biochem. J. 221, 471–475 (1984). In references 21–23, the research group of Karl Brand provides some of the first evidence, for lymphocytes, that most of the glucose imported into quiescent rat thymocytes is fully oxidized to carbon dioxide, whereas proliferating thymocytes excrete large amounts of lactate.
Buttgereit, F., Brand, M. D. & Muller, M. ConA induced changes in energy metabolism of rat thymocytes. Biosci. Rep. 12, 381–386 (1992).
Buttgereit, F. & Brand, M. D. A hierarchy of ATP-consuming processes in mammalian cells. Biochem. J. 312, 163–167 (1995).
Frauwirth, K. A. et al. The CD28 signaling pathway regulates glucose metabolism. Immunity 16, 769–777 (2002).
Perl, A., Gergely, P. Jr, Puskas, F. & Banki, K. Metabolic switches of T-cell activation and apoptosis. Antioxid. Redox Signal. 4, 427–443 (2002).
Brand, K. et al. Cell-cycle-related metabolic and enzymatic events in proliferating rat thymocytes. Eur. J. Biochem. 172, 695–702 (1988).
Buzzai, M. et al. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid β-oxidation. Oncogene 24, 4165–4173 (2005).
Jones, R. G. et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell 18, 283–293 (2005).
Miller, E. S., Klinger, J. C., Akin, C., Koebel, D. A. & Sonnenfeld, G. Inhibition of murine splenic T lymphocyte proliferation by 2-deoxy-D-glucose-induced metabolic stress. J. Neuroimmunol. 52, 165–173 (1994).
MacDonald, H. R. & Cerottini, J. C. Inhibition of T cell-mediated cytolysis by 2-deoxy-D-glucose (2-DG): differential effect of 2-DG on effector cells isolated early or late after alloantigenic stimulation in vitro. J. Immunol. 122, 1067–1072 (1979). Both references 31 and 32 provide evidence that affecting glycolysis (using the non-metabolizable analogue 2-deoxyglucose) can adversely affect the T-cell-dependent immune response by inhibiting proliferation.
Inoki, K., Corradetti, M. N. & Guan, K. L. Dysregulation of the TSC–mTOR pathway in human disease. Nature Genet. 37, 19–24 (2005).
Song, G., Ouyang, G. & Bao, S. The activation of Akt/PKB signaling pathway and cell survival. J. Cell. Mol. Med. 9, 59–71 (2005).
Carling, D. AMP-activated protein kinase: balancing the scales. Biochimie 87, 87–91 (2005).
Sekulic, A. et al. A direct linkage between the phosphoinositide 3-kinase–AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 60, 3504–3513 (2000).
Li, Y., Corradetti, M. N., Inoki, K. & Guan, K. L. TSC2: filling the GAP in the mTOR signaling pathway. Trends Biochem. Sci. 29, 32–38 (2004).
Edinger, A. L., Linardic, C. M., Chiang, G. G., Thompson, C. B. & Abraham, R. T. Differential effects of rapamycin on mammalian target of rapamycin signaling functions in mammalian cells. Cancer Res. 63, 8451–8460 (2003).
Sarbassov, D. D., Guertin, D. A., Ali, S. M. & Sabatini, D. M. Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science 307, 1098–1101 (2005).
Plas, D. R., Talapatra, S., Edinger, A. L., Rathmell, J. C. & Thompson, C. B. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J. Biol. Chem. 276, 12041–12048 (2001).
Lizcano, J. M. et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 23, 833–843 (2004).
Hawley, S. A. et al. Complexes between the LKB1 tumor suppressor, STRAD α/β and MO25 α/β are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. [online] 2, 28 (2003).
Wilson, W. A. & Roach, P. J. Nutrient-regulated protein kinases in budding yeast. Cell 111, 155–158 (2002).
Hardie, D. G., Carling, D. & Carlson, M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 67, 821–855 (1998).
Jhun, B. S. et al. AICAR suppresses IL-2 expression through inhibition of GSK-3 phosphorylation and NF-AT activation in Jurkat T cells. Biochem. Biophys. Res. Commun. 332, 339–346 (2005).
Stefanelli, C. et al. Inhibition of glucocorticoid-induced apoptosis with 5-aminoimidazole-4-carboxamide ribonucleoside, a cell-permeable activator of AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 243, 821–826 (1998).
Princiotta, M. F. et al. Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity 18, 343–354 (2003). In this study, the authors provide a quantitative assessment of the energy cost of regulated protein turnover, using MHC-class-I-dependent antigen presentation in murine L929 and myeloid cells as a model system.
Yewdell, J. W. Not such a dismal science: the economics of protein synthesis, folding, degradation and antigen processing. Trends Cell Biol. 11, 294–297 (2001). This is a Review on the topic of reference 47 by the same research group.
Hay, N. & Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 18, 1926–1945 (2004).
Edinger, A. L. & Thompson, C. B. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol. Biol. Cell 13, 2276–2288 (2002).
Kanai, Y. & Hediger, M. A. The glutamate and neutral amino acid transporter family: physiological and pharmacological implications. Eur. J. Pharmacol. 479, 237–247 (2003).
Edinger, A. L., Cinalli, R. M. & Thompson, C. B. Rab7 prevents growth factor-independent survival by inhibiting cell-autonomous nutrient transporter expression. Dev. Cell 5, 571–582 (2003).
Kimball, S. R. & Jefferson, L. S. Regulation of global and specific mRNA translation by oral administration of branched-chain amino acids. Biochem. Biophys. Res. Commun. 313, 423–427 (2004).
Reiter, A. K., Anthony, T. G., Anthony, J. C., Jefferson, L. S. & Kimball, S. R. The mTOR signaling pathway mediates control of ribosomal protein mRNA translation in rat liver. Int. J. Biochem. Cell Biol. 36, 2169–2179 (2004).
Mordier, S., Deval, C., Bechet, D., Tassa, A. & Ferrara, M. Leucine limitation induces autophagy and activation of lysosome-dependent proteolysis in C2C12 myotubes through a mammalian target of rapamycin-independent signaling pathway. J. Biol. Chem. 275, 29900–29906 (2000).
Proud, C. G. Role of mTOR signalling in the control of translation initiation and elongation by nutrients. Curr. Top. Microbiol. Immunol. 279, 215–244 (2004).
Land, S. C. & Hochachka, P. W. Protein turnover during metabolic arrest in turtle hepatocytes: role and energy dependence of proteolysis. Am. J. Physiol. 266, C1028–C1036 (1994). This paper describes a study that was carried out in turtle hepatocytes to quantify the portion of cellular ATP that is devoted to regulated protein degradation under aerobic and anaerobic conditions.
Liu, J. O. The yins of T cell activation. Sci STKE [online] 2005, re1 (2005). This is an excellent, recent review of the transcription factors that negatively regulate T-cell activation, several of which potentiate T-cell quiescence by promoting 'active' suppression of the cell-cycle machinery.
Lin, L., Hron, J. D. & Peng, S. L. Regulation of NF-κB, TH activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity 21, 203–213 (2004).
Buckley, A. F., Kuo, C. T. & Leiden, J. M. Transcription factor LKLF is sufficient to program T cell quiescence via a c-Myc-dependent pathway. Nature Immunol. 2, 698–704 (2001).
Kuo, C. T., Veselits, M. L. & Leiden, J. M. LKLF: a transcriptional regulator of single-positive T cell quiescence and survival. Science 277, 1986–1990 (1997).
Duan, L., Reddi, A. L., Ghosh, A., Dimri, M. & Band, H. The Cbl family and other ubiquitin ligases: destructive forces in control of antigen receptor signaling. Immunity 21, 7–17 (2004).
Dutcher, J. P. Mammalian target of rapamycin (mTOR) inhibitors. Curr. Oncol. Rep. 6, 111–115 (2004).
Grube, E. & Buellesfeld, L. Rapamycin analogs for stent-based local drug delivery. Everolimus- and tacrolimus-eluting stents. Herz 29, 162–166 (2004).
Ussetti, P., Carreno, M. C., de Pablo, A., Gamez, P. & Varela, A. Rapamycin and chronic lung rejection. J. Heart Lung Transplant. 23, 917–918 (2004).
Trosch, F. et al. First experience with rapamycin-based immunosuppression to improve kidney function after heart transplantation. Thorac. Cardiovasc. Surg. 52, 163–168 (2004).
Kay, J. E., Kromwel, L., Doe, S. E. & Denyer, M. Inhibition of T and B lymphocyte proliferation by rapamycin. Immunology 72, 544–549 (1991).
Luo, H., Duguid, W., Chen, H., Maheu, M. & Wu, J. The effect of rapamycin on T cell development in mice. Eur. J. Immunol. 24, 692–701 (1994).
Damoiseaux, J. G., Beijleveld, L. J., Schuurman, H. J. & van Breda Vriesman, P. J. Effect of in vivo rapamycin treatment on de novo T-cell development in relation to induction of autoimmune-like immunopathology in the rat. Transplantation 62, 994–1001 (1996).
Tian, L., Lu, L., Yuan, Z., Lamb, J. R. & Tam, P. K. Acceleration of apoptosis in CD4+CD8+ thymocytes by rapamycin accompanied by increased CD4+CD25+ T cells in the periphery. Transplantation 77, 183–189 (2004).
Fox, C. J., Hammerman, P. S. & Thompson, C. B. The Pim kinases control rapamycin-resistant T cell survival and activation. J. Exp. Med. 201, 259–266 (2005).
Battaglia, M., Stabilini, A. & Roncarolo, M. G. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 105, 4743–4748 (2005). This study provided the first clue that part of the immunosuppressive ability of rapamycin in vivo involves the selective expansion of CD4+CD25+ regulatory T-cell populations.
Fuge, E. K., Braun, E. L. & Werner-Washburne, M. Protein synthesis in long-term stationary-phase cultures of Saccharomyces cerevisiae. J. Bacteriol. 176, 5802–5813 (1994).
Barbet, N. C. et al. TOR controls translation initiation and early G1 progression in yeast. Mol. Biol. Cell 7, 25–42 (1996).
Thomsson, E., Svensson, M. & Larsson, C. Rapamycin pre-treatment preserves viability, ATP level and catabolic capacity during carbon starvation of Saccharomyces cerevisiae. Yeast 22, 615–623 (2005).
Rathmell, J. C., Farkash, E. A., Gao, W. & Thompson, C. B. IL-7 enhances the survival and maintains the size of naive T cells. J. Immunol. 167, 6869–6876 (2001).
Hammerman, P. S., Fox, C. J., Birnbaum, M. J. & Thompson, C. B. Pim and Akt oncogenes are independent regulators of hematopoietic cell growth and survival. Blood 105, 4477–4483 (2005).
Hammerman, P. S. et al. Lymphocyte transformation by Pim-2 is dependent on nuclear factor-κB activation. Cancer Res. 64, 8341–8348 (2004).
Fox, C. J. et al. The serine/threonine kinase Pim-2 is a transcriptionally regulated apoptotic inhibitor. Genes Dev. 17, 1841–1854 (2003).
Bachmann, M. & Moroy, T. The serine/threonine kinase Pim-1. Int. J. Biochem. Cell Biol. 37, 726–730 (2005).
Jacobs, M. D. et al. Pim-1 ligand-bound structures reveal the mechanism of serine/threonine kinase inhibition by LY294002. J. Biol. Chem. 280, 13728–13734 (2005).
Qian, K. C. et al. Structural basis of constitutive activity and a unique nucleotide binding mode of human Pim-1 kinase. J. Biol. Chem. 280, 6130–6137 (2005).
Kumar, A. et al. Crystal structures of proto-oncogene kinase Pim1: a target of aberrant somatic hypermutations in diffuse large cell lymphoma. J. Mol. Biol. 348, 183–193 (2005).
Leduc, I. et al. The Pim-1 kinase stimulates maturation of TCRβ-deficient T cell progenitors: implications for the mechanism of Pim-1 action. Int. Immunol. 12, 1389–1396 (2000).
Pearson, R. & Weston, K. c-Myb regulates the proliferation of immature thymocytes following β-selection. EMBO J. 19, 6112–6120 (2000).
Angelini, G. et al. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc. Natl Acad. Sci. USA 99, 1491–1496 (2002).
Droge, W., Eck, H. P., Gmunder, H. & Mihm, S. Modulation of lymphocyte functions and immune responses by cysteine and cysteine derivatives. Am. J. Med. 91, 140S–144S (1991).
Gmunder, H., Eck, H. P. & Droge, W. Low membrane transport activity for cystine in resting and mitogenically stimulated human lymphocyte preparations and human T cell clones. Eur. J. Biochem. 201, 113–117 (1991).
Edinger, A. L. & Thompson, C. B. Antigen-presenting cells control T cell proliferation by regulating amino acid availability. Proc. Natl Acad. Sci. USA 99, 1107–1109 (2002).
Gmunder, H., Eck, H. P., Benninghoff, B., Roth, S. & Droge, W. Macrophages regulate intracellular glutathione levels of lymphocytes. Evidence for an immunoregulatory role of cysteine. Cell. Immunol 129, 32–46 (1990).
Bauer, T. M. et al. Studying the immunosuppressive role of indoleamine 2,3-dioxygenase: tryptophan metabolites suppress rat allogeneic T-cell responses in vitro and in vivo. Transpl. Int. 18, 95–100 (2005).
Munn, D. H., Sharma, M. D. & Mellor, A. L. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J. Immunol. 172, 4100–4110 (2004).
Mellor, A. L. et al. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int. Immunol. 16, 1391–1401 (2004).
Munn, D. H. et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 22, 633–642 (2005). Recently, the induction of IDO expression in activated dendritic cells was found to suppress T-cell proliferation by activating the stress-responsive kinase GCN2. GCN2 is also activated by amino-acid withdrawal and can affect both cap-dependent and -independent translation by regulating EIF2α phosphorylation.
Grossman, Z., Min, B., Meier-Schellersheim, M. & Paul, W. E. Concomitant regulation of T-cell activation and homeostasis. Nature Rev. Immunol. 4, 387–395 (2004).
Seder, R. A., Germain, R. N., Linsley, P. S. & Paul, W. E. CD28-mediated costimulation of interleukin 2 (IL-2) production plays a critical role in T cell priming for IL-4 and interferon γ production. J. Exp. Med. 179, 299–304 (1994).
Sallusto, F., Geginat, J. & Lanzavecchia, A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22, 745–763 (2004).
Rocha, B. & Tanchot, C. Towards a cellular definition of CD8+ T-cell memory: the role of CD4+ T-cell help in CD8+ T-cell responses. Curr. Opin. Immunol. 16, 259–263 (2004).
Fontenot, J. D. & Rudensky, A. Y. Molecular aspects of regulatory T cell development. Semin. Immunol. 16, 73–80 (2004).
Fehervari, Z. & Sakaguchi, S. Development and function of CD25+CD4+ regulatory T cells. Curr. Opin. Immunol. 16, 203–208 (2004).
Bayer, A. L., Yu, A., Adeegbe, D. & Malek, T. R. Essential role for interleukin-2 for CD4+CD25+ T regulatory cell development during the neonatal period. J. Exp. Med. 201, 769–777 (2005).
Vivanco, I. & Sawyers, C. L. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nature Rev. Cancer 2, 489–501 (2002).
Acknowledgements
We thank J. Gruber and T. Lindsten for their critical assessment of the manuscript. C.J.F. is supported by a fellowship from The Leukemia & Lymphoma Society (United States). P.S.H. is a recipient of a Pre-doctoral Training Grant from the Cancer Research Institute (United States). Funding to support the laboratory of C.B.T. is provided, in part, by the National Institutes of Health (United States).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- GLYCOLYSIS
-
A metabolic process that occurs in the cytosol and breaks down one molecule of glucose into two molecules of pyruvate, resulting in the production of ATP. Pyruvate is converted to lactate, which regenerates the NAD that is required as an electron acceptor in this catabolic process. Alternatively, pyruvate can be oxidized in the tricarboxylic-acid cycle, and NAD can be regenerated in one of two mitochondrial shuttles that end with electron donation to the electron-transport chain.
- OXIDATIVE PHOSPHORYLATION
-
A metabolic process that encompasses two sets of reactions. The first reaction involves the conversion of intermediate molecules (pyruvate and fatty acids) to acetyl coenzyme A (acetyl-CoA) and the degradation of acetyl-CoA to carbon dioxide in the tricarboxylic-acid cycle, yielding free electrons that are carried by NADH and FADH2. The second reaction involves the transfer of electrons from NADH and FADH2 to the electron-transport chain, resulting in the movement of protons out of the mitochondrial matrix. The resulting electrochemical potential is used by the F1F0 ATP synthase to synthesize ATP.
- CATABOLIC METABOLISM
-
The breakdown of complex substances into simpler ones. This often allows cells to capture the released reduction equivalents and channel them into ATP production. Examples include the oxidation of fatty acids and amino acids.
- ANABOLIC METABOLISM
-
The synthesis of complex macromolecules from simpler intermediates at the expense of ATP. Examples include the synthesis of nucleotides from ribose-5-phosphate, lipids from acetyl coenzyme A, and proteins from amino acids.
- AUTOPHAGY
-
An evolutionarily conserved process in which acidic double-membrane vacuoles sequester intracellular contents (such as damaged organelles and macromolecules) and target them for degradation, through fusion to secondary lysosomes.
- AEROBIC GLYCOLYSIS
-
A metabolic process that is preferentially induced in proliferating lymphocytes and is also characteristic of neoplastic and transformed cells. In contrast to anaerobic conditions (such as in skeletal muscle and inflamed tissues), in which glycolysis is the main source of ATP, lymphocytes in the peripheral blood are not exposed to low oxygen concentrations; however, they continue to excrete excess pyruvate as lactate rather than fully oxidizing it in the tricarboxylic-acid cycle.
- NECROSIS
-
Forms of cell death that result from a decline in cellular ATP or ADP levels below the concentration that is required to maintain cellular organization and integrity.
- β-OXIDATION
-
The oxidative breakdown of fatty acids into acetyl coenzyme A (acetyl-CoA) by stepwise oxidation at the β-carbon atom. Each round of oxidation yields one NADH molecule and one FADH2 molecule, and the acetyl-CoA that is also produced enters the tricarboxylic-acid cycle.
- CAP-DEPENDENT TRANSLATION
-
A mechanism for initiation of translation. It involves the binding of the EIF4F complex (which is composed of the mRNA helicase EIF4A (eukaryotic translation-initiation factor 4A), EIF4E, the scaffolding protein EIF4G and poly(A)-binding protein) to mRNA transcripts with a 5′ 7-methyl guanylate cap, resulting in recruitment of the 43S ribosome. The ribosome then scans the mRNA until it reaches the first AUG codon, and it initiates translation following the GTP-dependent release of EIF2B.
- E3 UBIQUITIN LIGASE
-
A molecule that has a key role in protein turnover. It recognizes substrate proteins with a high degree of specificity and targets them to the ubiquitin-dependent proteasomal machinery. E3 ubiquitin ligases — including CBL (Casitas B-lineage lymphoma), ITCH and NEDD4 (neural precursor-cell expressed, developmentally downregulated 4) — are crucial regulators of the T-cell response to pro-survival and mitogenic signals.
- CAP-INDEPENDENT TRANSLATION
-
The ability of certain mRNA transcripts to directly recruit the 43S ribosome without previous formation of a 5′ 7methyl guanylate cap. One way that this occurs is by internal ribosomal-entry sites (IRESs) that can directly interact with the 43S ribosome. IRES-dependent translation is one way that viruses can co-opt the cellular translation machinery to drive viral replication.
Rights and permissions
About this article
Cite this article
Fox, C., Hammerman, P. & Thompson, C. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol 5, 844–852 (2005). https://doi.org/10.1038/nri1710
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri1710
This article is cited by
-
The immune checkpoint TIGIT/CD155 promotes the exhaustion of CD8 + T cells in TNBC through glucose metabolic reprogramming mediated by PI3K/AKT/mTOR signaling
Cell Communication and Signaling (2024)
-
Metabolism Serves as a Bridge Between Cardiomyocytes and Immune Cells in Cardiovascular Diseases
Cardiovascular Drugs and Therapy (2024)
-
Epigenetic reprogramming of T cells: unlocking new avenues for cancer immunotherapy
Cancer and Metastasis Reviews (2024)
-
Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions
Signal Transduction and Targeted Therapy (2023)
-
Better prediction of clinical outcome in clear cell renal cell carcinoma based on a 6 metabolism-related gene signature
Scientific Reports (2023)