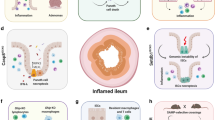
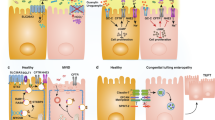
Key Points
-
Substantial progress has been made in the understanding of IBD immunopathogenesis during the past few decades
-
Discovery of the cellular and molecular mediators of intestinal inflammation has led to the development of new therapies that clearly have benefited patients with IBD
-
Environmental, genetic and microbial factors interact with the immune system, resulting in dysregulated immune responses responsible for chronic intestinal inflammation typical of Crohn's disease and ulcerative colitis
-
IBD pathogenesis also includes the effects of other cells involved in the inflammatory processes (such as epithelial, endothelial, mesenchymal and fat cells), as well as other components (such as the inflammasome and regulatory RNAs)
-
Immunopathogenic events are only one component of IBD and they must be interpreted in the context of the other components; that is, the environment, genome and microbiota
-
Only the functional integration of all the underlying components will lead to a full understanding and cure of IBD
Abstract
IBD is a chronic inflammatory condition of the gastrointestinal tract encompassing two main clinical entities: Crohn's disease and ulcerative colitis. Although Crohn's disease and ulcerative colitis have historically been studied together because they share common features (such as symptoms, structural damage and therapy), it is now clear that they represent two distinct pathophysiological entities. Both Crohn's disease and ulcerative colitis are associated with multiple pathogenic factors including environmental changes, an array of susceptibility gene variants, a qualitatively and quantitatively abnormal gut microbiota and a broadly dysregulated immune response. In spite of this realization and the identification of seemingly pertinent environmental, genetic, microbial and immune factors, a full understanding of IBD pathogenesis is still out of reach and, consequently, treatment is far from optimal. An important reason for this unsatisfactory situation is the currently limited comprehension of what are the truly relevant components of IBD immunopathogenesis. This article will comprehensively review current knowledge of the classic immune components and will expand the concept of IBD immunopathogenesis to include various cells, mediators and pathways that have not been traditionally associated with disease mechanisms, but that profoundly affect the overall intestinal inflammatory process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bernstein, C. N. & Shanahan, F. Disorders of a modern lifestyle: reconciling the epidemiology of inflammatory bowel diseases. Gut 57, 1185–1191 (2008).
Ng, S. C. et al. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 62, 630–649 (2013).
Hviid, A., Svanstrom, H. & Frisch, M. Antibiotic use and inflammatory bowel diseases in childhood. Gut 60, 49–54 (2011).
Garcia-Rodriguez, L. A., Ruigomez, A. & Panes, J. Acute gastroenteritis is followed by an increased risk of inflammatory bowel disease. Gastroenterology 130, 1588–1594 (2006).
Benjamin, J. L. et al. Smokers with active Crohn's disease have a clinically relevant dysbiosis of the gastrointestinal microbiota. Inflamm. Bowel. Dis. 18, 1092–1100 (2012).
Nickerson, K. P. & McDonald, C. Crohn's disease-associated adherent-invasive Escherichia coli adhesion is enhanced by exposure to the ubiquitous dietary polysaccharide maltodextrin. PLoS ONE 7, e52132 (2012).
Nerich, V. et al. Low exposure to sunlight is a risk factor for Crohn's disease. Aliment. Pharmacol. Ther. 33, 940–945 (2011).
Boneberger, A. et al. Endotoxin levels in house dust samples and juvenile inflammatory bowel disease – a case–control study. J. Crohns Colitis 5, 525–530 (2011).
Rook, G. A. Hygiene hypothesis and autoimmune diseases. Clin. Rev. Allergy Immunol. 42, 5–15 (2012).
Chen, Y. & Blaser, M. J. Helicobacter pylori colonization is inversely associated with childhood asthma. J. Infect. Dis. 198, 553–560 (2008).
Matsushima, K. & Nagai, S. Unraveling the mystery of the hygiene hypothesis through Helicobacter pylori infection. J. Clin. Invest. 122, 801–804 (2012).
Saidel-Odes, L. & Odes, S. Hygiene hypothesis in inflammatory bowel disease. Ann. Gastroenterol. 27, 189–190 (2014).
Inoue, N. et al. Lack of common NOD2 variants in Japanese patients with Crohn's disease. Gastroenterology 123, 86–91 (2002).
Leong, R. W. et al. NOD2/CARD15 gene polymorphism and Crohn's disease in the Chinese population. Aliment. Pharmacol. Ther. 17, 1465–1470 (2003).
Duerr, R. H. et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314, 1461–1463 (2006).
Rivas, M. A. et al. Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat. Genet. 43, 1066–1073 (2011).
Jostins, L. et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012).
Shaw, M. H., Kamada, N., Warner, N., Kim, Y. G. & Nunez, G. The ever-expanding function of NOD2: autophagy, viral recognition, and T cell activation. Trends Immunol. 32, 73–79 (2011).
Noguchi, E., Homma, Y., Kang, X., Netea, M. G. & Ma, X. A Crohn's disease-associated NOD2 mutation suppresses transcription of human IL10 by inhibiting activity of the nuclear ribonucleoprotein hnRNP-A1. Nat. Immunol. 10, 471–479 (2009).
Hampe, H. et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat. Genet. 39, 207–211 (2007).
Parkes, M. et al. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn's disease susceptibility. Nat. Genet. 39, 830–832 (2007).
Hoefkens, E. et al. Genetic association and functional role of Crohn disease risk alleles involved in microbial sensing, autophagy, and endoplasmic reticulum (ER) stress. Autophagy 9, 2046–2055 (2013).
Levine, B., Mizushima, N. & Virgin, H. W. Autophagy in immunity and inflammation. Nature 469, 323–335 (2011).
Homer, C. R., Richmond, A. L., Rebert, N. A., Achkar, J. P. & McDonald, C. ATG16L1 and NOD2 interact in an autophagy-dependent antibacterial pathway implicated in Crohn's disease pathogenesis. Gastroenterology 139, 1630–1641.e1–e2 (2010).
Stappenbeck, T. S. et al. Crohn disease: a current perspective on genetics, autophagy and immunity. Autophagy 7, 355–374 (2011).
Schwab, M. et al. Association between the C3435T MDR1 gene polymorphism and susceptibility for ulcerative colitis. Gastroenterology 124, 26–33 (2003).
Wang, J. et al. MDR1 C3435T polymorphism and inflammatory bowel disease risk: a meta-analysis. Mol. Biol. Rep. 41, 79–85 (2014).
Franchimont, D. et al. Deficient host-bacteria interactions in inflammatory bowel disease? The toll-like receptor (TLR)-4 Asp299gly polymorphism is associated with Crohn's disease and ulcerative colitis. Gut 53, 987–992 (2004).
Kaser, A. et al. XBP1 links ER stress to intestinal inflammation and confers genetci risk for human inflammatory bowel disease. Cell 134, 743–756 (2008).
Villani, A. C. et al. Common variants in the NLRP3 region contribute to Crohn's disease susceptibility. Nat. Genet. 41, 71–76 (2009).
Venema, K. Role of gut microbiota in the control of energy and carbohydrate metabolism. Curr. Opin. Clin. Nutr. Metab. Care 13, 432–438 (2010).
Dominguez-Bello, M. G., Blaser, M. J., Ley, R. E. & Knight, R. Development of the human gastrointestinal microbiota and insights from high-throughput sequencing. Gastroenterology 140, 1713–1719 (2011).
Lathrop, S. K. et al. Peripheral education of the immune system by colonic commensal microbiota. Nature 478, 250–254 (2011).
Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 449, 819–826 (2007).
Hooper, L. V., Littman, D. R. & Macpherson, A. J. Interactions between the microbiota and the immune system. Science 336, 1268–1273 (2012).
Quinton, J. F. et al. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut 42, 788–791 (1998).
Mow, W. S. et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn's disease. Gastroenterology 126, 414–424 (2004).
Lodes, M. J. et al. Bacterial flagellin is a dominant antigen in Crohn disease. J. Clin. Invest. 113, 1296–1306 (2004).
Murdoch, T. B. et al. Pattern recognition receptor and autophagy gene variants are associated with development of antimicrobial antibodies in Crohn's disease. Inflamm. Bowel Dis. 18, 1743–1748 (2012).
Dotan, I. et al. Antibodies against laminaribioside and chitobioside are novel serologic markers in Crohn's disease. Gastroenterology 131, 366–378 (2006).
Pirzer, U., Schonhaar, A., Fleischer, B., Hermann, E. & MeyerzumBuschenfelde, K.-H. Reactivity of infiltrating T lymphocytes with microbial antigens in Crohn's disease. Lancet 338, 1238–1239 (1991).
D'Haens, G. et al. Early lesions caused by infusion of intestinal contents in excluded ileum of Crohn's disease. Gastroenterology 114, 262–267 (1998).
Chassaing, B. & Darfeuille-Michaud, A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology 140, 1720–1728 (2011).
Man, S. M., Kaakoush, N. O. & Mitchell, H. M. The role of bacteria and pattern-recognition receptors in Crohn's disease. Nat. Rev. Gastroenterol. Hepatol. 8, 152–168 (2011).
Hansen, R. et al. Microbiota of de-novo pediatric IBD: increased Faecalibacterium prausnitzii and reduced bacterial diversity in Crohn's but not in ulcerative colitis. Am. J. Gastroenterol. 107, 1913–1922 (2012).
Andoh, A. et al. Comparison of the fecal microbiota profiles between ulcerative colitis and Crohn's disease using terminal restriction fragment length polymorphism analysis. J. Gastroenterol. 46, 479–486 (2011).
Mazmanian, S. K., Round, J. L. & Kasper, D. L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453, 620–625 (2008).
Sokol, H. et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl Acad. Sci. USA 105, 16731–16736 (2008).
Gevers, D. et al. The treatment-naive microbiome in new-onset Crohn's disease. Cell Host Microbe 15, 382–392 (2014).
Sewell, G. W., Marks, D. J. & Segal, A. W. The immunopathogenesis of Crohn's disease: a three-stage model. Curr. Opin. Immunol. 21, 506–513 (2009).
Fritz, T., Niederreiter, L., Adolph, T., Blumberg, R. S. & Kaser, A. Crohn's disease: NOD2, autophagy and ER stress converge. Gut 60, 1580–1588 (2011).
Kaser, A. & Blumberg, R. S. Endoplasmic reticulum stress and intestinal inflammation. Mucosal Immunol. 3, 11–16 (2010).
McGovern, D. P. B. et al. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat. Genet. 42, 332–337 (2010).
Mukherjee, P. K. et al. Mycobiota in gastrointestinal diseases. Nat. Rev. Gastroenterol. Hepatol. 12, 77–87 (2015).
Norman, J. M. et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell 160, 447–460 (2015).
Rausch, P. et al. Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc. Natl Acad. Sci. USA 108, 19030–19035 (2011).
Lupp, C. et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2, 119–129 (2007).
Bruce, A., Black, M. & Bhattacharya, S. Mode of delivery and risk of inflammatory bowel disease in the offspring: systematic review and meta-analysis of observational studies. Inflamm. Bowel Dis. 20, 1217–1226 (2014).
Kobayashi, K. S. et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307, 731–734 (2005).
Wehkamp, J. et al. Inducible and constitutive β-defensins are differentially expressed in Crohn's disease and ulcerative colitis. Inflamm. Bowel Dis. 9, 215–223 (2003).
Wehkamp, J. et al. Reduced Paneth cell α-defensins in ileal Crohn's disease. Proc. Natl Acad. Sci. USA 102, 18129–18134 (2005).
Billmann-Born, S. et al. The complex interplay of NOD-like receptors and the autophagy machinery in the pathophysiology of Crohn disease. Eur. J. Cell Biol. 90, 593–602 (2011).
Lapaquette, P., Glasser, A. L., Huett, A., Xavier, R. J. & Darfeuille-Michaud, A. Crohn's disease-associated adherent-invasive, E. coli are selectively favoured by impaired autophagy to replicate intracellularly. Cell Microbiol. 12, 99–113 (2010).
Brazil, J. C., Louis, N. A. & Parkos, C. A. The role of polymorphonuclear leukocyte trafficking in the perpetuation of inflammation during inflammatory bowel disease. Inflamm. Bowel Dis. 19, 1556–1565 (2013).
Wynn, T. A., Chawla, A. & Pollard, J. W. Macrophage biology in development, homeostasis and disease. Nature 496, 445–455 (2013).
Martinez, F. O. & Gordon, S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6, 13 (2014).
Smythies, L. E. et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Invest. 115, 1066–1075 (2005).
Kamada, N. et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-γ axis. J. Clin. Invest. 118, 2269–2280 (2008).
Smith, A. M. et al. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn's disease. J. Exp. Med. 206, 1883–1897 (2009).
Rossi, M. & Young, J. W. Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J. Immunol. 175, 1373–1381 (2005).
Rescigno, M. & diSabatino, A. Dendritic cells in intestinal homeostasis and disease. J. Clin. Invest. 119, 2441–2450 (2009).
Rimoldi, M. et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat. Immunol. 6, 507–514 (2005).
Hart, A. L. et al. Characteristics of intestinal dendritic cells in inflammatory bowel disease. Gastroenterology 129, 50–65 (2005).
Middel, P., Raddatz, D., Gunawan, B., Haller, F. & Radzun, H. J. Increased number of mature dendritic cells in Crohn's disease: evidence for a chemokine mediated retention mechanism. Gut 55, 220–227 (2006).
Scott, M. G. et al. Spontaneous secretion of IgG subclasses by intestinal mononuclear cells: differences between ulcerative colitis, Crohn's disease, and controls. Clin. Exp. Immunol. 66, 209–215 (1986).
MacDermott, R. P., Nash, G. S. & Nahm, M. H. Antibody secretion by human intestinal mononuclear cells from normal controls and inflammatory bowel disease patients. Immunol. Invest. 18, 449–457 (1989).
Takahashi, F. & Das, K. M. Isolation and characterization of a colonic autoantigen specifically recognized by colon tissue-bound immunoglobulin G. from idiopathic ulcerative colitis. J. Clin. Invest. 76, 311–318 (1985).
Das, K. M., Vecchi, M. & Sakamaki, S. A shared and unique epitope(s) on human colon, skin, and biliary epithelium detected by a monoclonal antibody. Gastroenterology 98, 464–469 (1990).
Halstensen, T. S., Das, K. M. & Brandtzaeg, P. Epithelial deposits of immunoglobulin G1 and activated complement colocalise with the Mr 40kD putative autoantigen in ulcerative colitis. Gut 34, 650–657 (1993).
Geng, X. et al. Tropomyosin isoform in intestinal mucosa: production of autoantibodies to tropomyosin isoforms in ulcerative colitis. Gastroenterology 114, 912–922 (1998).
Duerr, R. H. et al. Neutrophil cytoplasmic antibodies: a link between sclerosing cholangitis and ulcerative colitis. Gastroenterology 100, 1385–1391 (1991).
McKenzie, H., Main, J., Pennington, C. R. & Parratt, D. Antibody to selected strains of Saccharomyces cerevisiae (baker's and brewer' yeast) and Candida albicans in Crohn's disease. Gut 31, 536–538 (1990).
Murphy, K. M. & Stockinger, B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat. Immunol. 11, 674–680 (2010).
O'Connor, W., Zenewicz, L. A. & Flavell, R. A. The dual function of TH17 cells: shifting the focus to function. Nat. Immunol. 11, 471–476 (2010).
Weaver, C. T. & Hatton, R. D. Interplay between the TH17 and Treg cell lineages: a (co-)evolutionary perspective. Nat. Rev. Immunol. 9, 883–889 (2009).
Berg, D. J. et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. J. Clin. Invest. 98, 1010–1020 (1996).
Becker, C. et al. Cutting edge: IL-23 cross-regulates IL-12 production in T cell-dependent experimental colitis. J. Immunol. 177, 2760–2764 (2006).
Annunziato, F. et al. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 204, 1849–1861 (2007).
Fujino, S. et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 52, 65–70 (2003).
Monteleone, G. et al. Interleukin-21 enhances T-helper cell type I signaling and interferon-gamma production in Crohn's disease. Gastroenterology 128, 687–694 (2005).
Fina, D. et al. Regulation of gut inflammation and TH17 cell response by interleukin-21. Gastroenterology 134, 1038–1048 (2008).
Brand, S. et al. IL-22 is increased in active Crohn's disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G827–G838 (2006).
Leung, J. M. et al. IL-22-producing CD4+ cells are depleted in actively inflamed colitis tissue. Mucosal Immunol. 7, 124–133 (2014).
Sugimoto, K. et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 118, 534–544 (2008).
van Beelen, A. J. et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity 27, 660–669 (2007).
Gerlach, K. et al. TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat. Immunol. 15, 676–686 (2014).
Harrison, O. J. & Powrie, F. M. Regulatory T cells and immune tolerance in the intestine. Cold Spring Harb. Perspect. Biol. 5, a018341 (2013).
Mayne, C. G. & Williams, C. B. Induced and natural regulatory T cells in the development of inflammatory bowel disease. Inflamm. Bowel Dis. 19, 1772–1788 (2013).
Mottet, C., Uhlig, H. H. & Powrie, F. Cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 170, 3939–3943 (2003).
Maul, J. et al. Peripheral and intestinal regulatory CD4+CD25+high T cells in inflammatory bowel disease. Gastroenterology 128, 1868–1878 (2005).
Makita, S. et al. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J. Immunol. 173, 3119–3130 (2004).
Huibregtse, I. L., vanLent, A. U. & van Deventer, S. J. H. Immunopathogenesis of IBD: insufficient suppressor function in the gut? Gut 56, 584–592 (2007).
Valencia, X. et al. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood 108, 253–261 (2006).
Ricciardelli, I., Lindley, K. J., Londei, M. & Quaratino, S. Anti tumour necrosis-alpha therapy increases the number of FOXP3 regulatory T cells in children affected by Crohn's disease. Immunology 125, 178–183 (2008).
Veltkamp, C. et al. Apoptosis of regulatory T lymphocytes is increased in chronic inflammatory bowel disease and reversed by anti-TNFα treatment. Gut 60, 1345–1353 (2011).
Kanai, T., Mikami, Y., Sujino, T., Hisamatsu, T. & Hibi, T. RORγt-dependent IL-17A-producing cells in the pathogenesis of intestinal inflammation. Mucosal Immunol. 5, 240–7 (2012).
Mizuno, S. et al. Cross-talk between RORγt+ innate lymphoid cells and intestinal macrophages induces mucosal IL-22 production in Crohn's disease. Inflamm. Bowel Dis. 20, 1426–1434 (2014).
Takayama, T. et al. Imbalance of NKp44+NKp46− and NKp44−NKp46+ natural killer cells in the intestinal mucosa of patients with Crohn's disease. Gastroenterology 139, 882–892.e1–e3 (2010).
Pariente, B. et al. Activation of the receptor NKG2D leads to production of Th17 cytokines in CD4+ T cells of patients with Crohn's disease. Gastroenterology 141, 217–226, 226 e1–e2 (2011).
Fuss, I. J. et al. IL-13Rα2-bearing, type II NKT cells reactive to sulfatide self-antigen populate the mucosa of ulcerative colitis. Gut 63, 1728–1736 (2014).
Buonocore, S. et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464, 1371–1375 (2010).
Geremia, A. et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J. Exp. Med. 208, 1127–1133 (2011).
Tait Wojno, E. D. & Artis, D. Innate lymphoid cells: balancing immunity, inflammation, and tissue repair in the intestine. Cell Host Microbe 12, 445–457 (2012).
Goldberg, R., Prescott, N., Lord, G. M., MacDonald, T. T. & Powell, N. The unusual suspects—innate lymphoid cells as novel therapeutic targets in IBD. Nat. Rev. Gastroenterol. Hepatol. 12, 271–283 (2015).
Strasser, A. & Pellegrini, M. T-lymphocyte death during shutdown of an immune response. Trends Immunol. 11, 610–615 (2004).
Boirivant, M. et al. Stimulated human lamina propria T cells manifest enhanced Fas-mediated apoptosis. J. Clin. Invest. 98, 2616–2622 (1996).
Ina, K. et al. Resistance of Crohn's disease T-cells to multiple apoptotic stimuli is associated with a Bcl-2/Bax mucosal imbalance. J. Immunol. 163, 1081–1090 (1999).
Sturm, A., Itoh, J., Jacobberger, J. W. & Fiocchi, C. p53 negatively regulates intestinal immunity by delaying mucosal T cell cycling. J. Clin. Invest. 109, 1481–1492 (2002).
Sturm, A. et al. Divergent cell cycle kinetics underlie the distinct functional capacity of mucosal T-cells in Crohn's disease (CD) and ulcerative colitis (UC). Gut 53, 1624–1631 (2004).
Atreya, R. et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat. Med. 6, 583–588 (2000).
de Souza, H. S. et al. Increased levels of survivin, via association with heat shock protein 90, in mucosal T cells from patients with Crohn's disease. Gastroenterology 143, 1017–1026.e9 (2012).
Tiede, I. et al. CD28-dependent Rac1 activation is the molecular target of azothioprine in primary human CD4+ T lymphocytes. J. Clin. Invest. 111, 1133–1145 (2003).
ten Hove, T., van Montfrans, C., Peppelenbosch, M. P. & van Deventer, S. J. H. Infliximab treatment induces apoptosis of lamina propria T-lymphocytes in Crohn's disease. Gut 50, 206–211 (2002).
Shen, C. et al. Adalimumab induces apoptosis of human monocytes: a comparative study with infliximab and etanercept. Aliment. Pharmacol. Ther. 21, 251–258 (2005).
Van den Brande, J. M. et al. Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn's disease. Gastroenterology 124, 1774–1785 (2003).
Nesbitt, A. et al. Mechanism of action of certolizumab (CDP870): in vitro comparison with other anti-tumor necrosis factors α agents. Inflamm. Bowel Dis. 13, 1323–1332 (2007).
Neurath, M. F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 14, 329–342 (2014).
Neurath, M. F. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 7, 6–19 (2014).
Fiocchi, C. & Podolsky, D. K. in Inflammatory Bowel Disease (eds Kirsner, J. B. & Shorter, R. G.) 252–280 (Williams & Wilkins, Baltimore, 1995).
Fuss, I. J. et al. Disparate CD4+ lamina propria lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-γ, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J. Immunol. 157, 1261–1270 (1996).
Monteleone, G. et al. Interleukin 12 is expressed and actively released by Crohn's disease intestinal lamina propria mononuclear cells. Gastroenterology 112, 1169–1178 (1997).
Pizarro, T. P. et al. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn's disease: expression and localization in intestinal mucosal cells. J. Immunol. 162, 6829–6835 (1999).
West, G. A., Matsuura, T., Levine, A. D., Klein, J. S. & Fiocchi, C. Interleukin-4 in inflammatory bowel disease and mucosal immune reactivity. Gastroenterology 110, 1683–1695 (1996).
Fuss, I. J. et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J. Clin. Invest. 113, 1490–1497 (2004).
Schreiber, S., Heinig, T., Thiele, H.-G. & Raedler, A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology 108, 1434–1444 (1995).
Autschbach, F. et al. In situ expression of interleukin-10 in noninflamed human gut and in inflammatory bowel disease. Am. J. Pathol. 153, 121–130 (1998).
Youngman, K. R. et al. Localization of intestinal interleukin 1 activity, protein and gene expression to lamina propria cells. Gastroenterology 104, 749–758 (1993).
Mudter, J. & Neurath, M. F. Apoptosis of T cells and the control of inflammatory bowel disease: therapeutic implications. Gut 56, 293–303 (2007).
Braegger, C. P., Nicholls, S., Murch, S. H., Stephens, S. & MacDonald, T. T. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet 339, 89–91 (1992).
Targan, S. R. et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor a for Crohn's disease. N. Engl. J. Med. 337, 1029–1035 (1997).
Li, M. O. & Flavell, R. A. TGF-beta: a master of all T cell trades. Cell 134, 392–404 (2008).
Monteleone, G. et al. Blocking Smad7 restores TGF-β1 signaling in chronic inflammatory bowel disease. J. Clin. Invest. 108, 601–609 (2001).
Monteleone, G. et al. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn's disease. N. Engl. J. Med. 372, 1104–1113 (2015).
Charo, I. F. & Ransohoff, R. M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354, 610–621 (2006).
Johnson, Z., Schwarz, M., Power, C. A., Wells, T. N. & Proudfoot, A. E. Multi-faceted strategies to combat disease by interference with the chemokine system. Trends Immunol. 26, 268–274 (2005).
Mahida, Y. R. et al. Enhanced synthesis of neutrophil-activating peptide-I/interleukin-8 in active ulcerative colitis. Clin. Sci. 82, 273–275 (1992).
Grimm, M. C. & Doe, W. F. Chemokines in inflammatory bowel disease mucosa: expression of RANTES, macrophage inflammatory protein (MIP)-1a, MIP-1b, and g-interferon-inducible protein 10 by macrophages, lymphocytes, endothelial cells, and granulomas. Inflamm. Bowel Dis. 2, 88–96 (1996).
Uguccioni, M. et al. Increased expression of IP-10, IL-8, MCP-1 and MCP-3 in ulcerative colitis. Am. J. Pathol. 155, 331–336 (1999).
Sans, M. et al. Enhanced recruitment of CX3CR1+ T-cells by mucosal endothelial cell-derived fractalkine in inflammatory bowel disease Gastroenterology 132, 139–153 (2007).
Papadakis, K. A. et al. CCR9-positive lymphocytes and thymus-expressed chemokine distinguish small bowel from colonic Crohn's disease. Gastroenterology 121, 246–254 (2001).
Kang, S. G. et al. Identification of a chemokine network that recruits FoxP3+ regulatory T cells into chronically inflamed intestine. Gastroenterology 132, 966–981 (2007).
Gebbers, J. O. & Otto, H. F. Alterations of the intestinal mucosal block in ulcerative colitis and Crohn's disease—immunological and ultrastructural findings, and considerations of the pathogenesis. Klin. Padiatr. 197, 341–348 (1985).
Mayer, L. & Shlien, R. Evidence for function of Ia molecules on gut epithelial cells in man. J. Exp. Med. 166, 1471–1483 (1987).
Mayer, L. & Eisenhardt, D. Lack of induction of suppressor T cells by intestinal epithelial cells from patients with inflammatory bowel disease. J. Clin. Invest. 86, 1255–1260 (1990).
Dubuquoy, L. et al. Impaired expression of peroxisome proliferator-activated receptor gamma in ulcerative colitis. Gastroenterology 124, 1538–1542 (2003).
Birchenough, G. M. H. et al. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 8, 712–719 (2015).
Jakobsson, H. E. et al. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 16, 164–177 (2015).
Van der Sluis, M. et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131, 117–129 (2006).
Buisine, M. P. et al. Abnormalities in mucin gene expression in Crohn's disease. Inflamm. Bowel Dis. 5, 24–32 (1999).
Johansson, M. E. et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 63, 281–291 (2014).
Uehara, A., Fujimoto, Y., Fukase, K. & Takada, H. Various human epithelial cells express functional Toll-like receptors, NOD1 and NOD2 to produce anti-microbial peptides, but not proinflammatory cytokines Mol. Immunol. 44, 3100–3111 (2007).
Turner, J. R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 (2009).
Bjarnason, I., O'Morain, C., Levi, A. J. & Peters, T. J. Absorption of 51-chromium-labelled ethylenediaminetetracetate in inflammatory bowel disease. Gastroenterology 85, 318–322 (1983).
Wyatt, J., Vogelsang, H., Hubl, W., Waldhoer, T. & Lochs, H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet 341, 1437–1439 (1993).
Hollander, D. et al. Increased intestinal permeability in patients with Crohn's disease and their relatives. Ann. Intern. Med. 105, 883–885 (1986).
Visser, J., Rozing, J., Sapone, A., Lammers, K. & Fasano, A. Tight junctions, intestinal permeability, and autoimmunity: celiac disease and type 1 diabetes paradigms. Ann. N. Y. Acad. Sci. 1165, 195–205 (2009).
Buhner, S. et al. Genetic basis for increased intestinal permeability in families with Crohn's disease: role of CARD15 3020insC mutation? Gut 55, 342–347 (2006).
Prager, M. et al. The JAK2 variant rs10758669 in Crohn's disease: altering the intestinal barrier as one mechanism of action. Int. J. Colorectal Dis. 27, 565–573 (2012).
Sheng, Y. H. et al. MUC1 and MUC13 differentially regulate epithelial inflammation in response to inflammatory and infectious stimuli. Mucosal Immunol. 6, 557–568 (2013).
Rieder, F. & Fiocchi, C. Intestinal fibrosis in IBD–a dynamic, multifactorial process. Nat. Rev. Gastroenterol. Hepatol. 6, 228–235 (2009).
Burke, J. P. et al. Fibrogenesis in Crohn's disease. Am. J. Gastroenterol. 102, 439–448 (2007).
Rieder, F., Zimmermann, E. M., Remzi, F. H. & Sandborn, W. J. Crohn's disease complicated by strictures: a systematic review. Gut 62, 1072–1084 (2013).
Gordon, I. O., Agrawal, N., Goldblum, J. R., Fiocchi, C. & Rieder, F. Fibrosis in ulcerative colitis: mechanisms, features, and consequences of a neglected problem. Inflamm. Bowel Dis. 20, 2198–2206 (2014).
Wynn, T. A. & Ramalingam, T. R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 18, 1028–1040 (2012).
Leeb, S. N. et al. Reduced migration of fibroblasts in inflammatory bowel disease: role of inflammatory mediators and focal adhesion kinase. Gastroenterology 125, 1341–1354 (2003).
Heuschkel, R. B. et al. Imbalance of stromelysin-1 and TIMP-1 in the mucosal lesions of children wih inflammatory bowel disease. Gut 47, 57–62 (2000).
Kirkegaard, T., Hansen, A., Bruun, E. & Brynskov, J. Expression and localisation of matrix metalloproteinases and their natural inhibitors in fistulae of patients with Crohn's disease. Gut 53, 701–709 (2004).
Speca, S., Giusti, I., Rieder, F. & Latella, G. Cellular and molecular mechanisms of intestinal fibrosis. World J. Gastroenterol. 18, 3635–3661 (2012).
Rieder, F. The gut microbiome in intestinal fibrosis: environmental protector or provocateur? Sci. Transl. Med. 5, 190ps10 (2013).
Danese, S. et al. Angiogenesis as a novel components of inflammatory bowel disease pathogenesis. Gastroenterology 130, 2060–2073 (2006).
Scaldaferri, F. et al. VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology 136, 585–595.e5 (2009).
Schirbel, A. et al. Pro-angiogenic activity of TLRs and NLRs: a novel link between gut microbiota and intestinal angiogenesis. Gastroenterology 144, 613–623 e9 (2013).
Danese, S. et al. Angiogenesis blockade as a new therapeutic approach to experimental colitis. Gut 56, 855–862 (2007).
Danese, S. et al. Platelets trigger a CD40-dependent inflammatory response in the microvasculature of inflammatory bowel disease patients. Gastroenterology 124, 1249–1264 (2003).
Van Kruiningen, H. J. & Colombel, J. F. The forgotten role of lymphangitis in Crohn's disease. Gut 57, 1–4 (2008).
Alitalo, K., Tammela, T. & Petrova, T. V. Lymphangiogenesis in development and human disease. Nature 438, 946–953 (2005).
Liao, S. & von der Weid, P. Y. Inflammation-induced lymphangiogenesis and lymphatic dysfunction. Angiogenesis 17, 325–334 (2014).
D'Alessio, S. et al. VEGF-C-dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J. Clin. Invest. 124, 3863–3878 (2014).
Kubota, Y. et al. Colonic vasoactive intestinal peptide nerves in inflammatory bowel disease. A digitized morphometric immunohistochemical study. Gastroenterology 102, 1242–1251 (1992).
Gross, K. J. & Pothoulakis, C. Role of neuropeptides in inflammatory bowel disease. Inflamm. Bowel Dis. 13, 918–932 (2007).
Bohorquez, D. V. & Liddle, R. A. The gut connectome: making sense of what you eat. J. Clin. Invest. 125, 888–890 (2015).
Kabouridis, P. S. & Pachnis, V. Emerging roles of gut microbiota and the immune system in the development of the enteric nervous system. J. Clin. Invest. 125, 956–964 (2015).
Mayer, E. A., Tillisch, K. & Gupta, A. Gut/brain axis and the microbiota. J. Clin. Invest. 125, 926–938 (2015).
Nathan, C. Epidemic inflammation: pondering obesity. Mol. Med. 14, 485–492 (2008).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Henao-Mejia, J., Elinav, E., Strowig, T. & Flavell, R. A. Inflammasomes: far beyond inflammation. Nat. Immunol. 13, 321–324 (2012).
Schaffler, A., Scholmerich, J. & Buchler, C. Mechanisms of disease: adipocytokines and visceral adipose tissue—emerging role in intestinal and mesenteric diseases. Nat. Clin. Pract. Gastroenterol. Hepatol. 2, 103–111 (2005).
Blain, A. et al. Crohn's disease clinical course and severity in obese patients. Clin. Nutr. 21, 51–57 (2002).
Uko, V. et al. Impact of abdominal visceral adipose tissue on disease outcome in pediatric Crohn's disease. Inflamm. Bowel Dis. 20, 2286–2269 (2014).
Paul, G. et al. Profiling adipocytokine secretion from creeping fat in Crohn's disease. Inflamm. Bowel Dis. 12, 471–477 (2006).
Peyrin-Biroulet, L. et al. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn's disease. Gut 61, 78–85 (2012).
Zulian, A. et al. Visceral adipocytes: old actors in obesity and new protagonists in Crohn's disease? Gut 61, 86–94 (2012).
Fink, C., Karagiannides, I., Bakirtzi, K. & Pothoulakis, C. Adipose tissue and inflammatory bowel disease pathogenesis. Inflamm. Bowel Dis. 18, 1550–1557 (2012).
Kotas, M. E. & Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 160, 816–827 (2015).
Strowig, T., Henao-Mejia, J., Elinav, E. & Flavell, R. Inflammasomes in health and disease. Nature 481, 278–286 (2012).
Lamkanfi, M. & Dixit, V. M. Mechanisms and functions of inflammasomes. Cell 157, 1013–1022 (2014).
Elinav, E., Henao-Mejia, J. & Flavell, R. A. Integrative inflammasome activity in the regulation of intestinal mucosal immune responses. Mucosal Immunol. 6, 4–13 (2013).
Bauer, C. et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 59, 1192–1199 (2010).
Zaki, M. H. et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32, 379–391 (2010).
Opipari, A. & Franchi, L. Role of Inflammasomes in Intestinal Inflammation and Crohn's Disease. Inflamm. Bowel Dis. 21, 173–181 (2015).
Rebane, A. & Akdis, C. A. MicroRNAs: Essential players in the regulation of inflammation. J. Allergy Clin. Immunol. 132, 15–26 (2013).
Tay, Y., Rinn, J. & Pandolfi, P. P. The multilayered complexity of ceRNA crosstalk and competition. Nature 505, 344–352 (2014).
Kalla, R. et al. MicroRNAs: new players in IBD. Gut 64, 504–517 (2015).
Wu, F. et al. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology 135, 1624–1635.e24 (2008).
Pekow, J. R. et al. miR-143 and miR-145 are downregulated in ulcerative colitis: putative regulators of inflammation and protooncogenes. Inflamm. Bowel Dis. 18, 94–100 (2012).
Wu, F. et al. Identification of microRNAs associated with ileal and colonic Crohn's disease. Inflamm. Bowel Dis. 16, 1729–1738 (2010).
Koukos, G. et al. MicroRNA-124 regulates STAT3 expression and is down-regulated in colon tissues of pediatric patients with ulcerative colitis. Gastroenterology 145, 842–852.e2 (2013).
Coskun, M., Bjerrum, J. T., Seidelin, J. B. & Nielsen, O. H. MicroRNAs in inflammatory bowel disease—pathogenesis, diagnostics and therapeutics. World J. Gastroenterol. 18, 4629–4634 (2012).
Rubartelli, A. & Lotze, M. T. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 28, 429–436 (2007).
Piccinini, A. M. & Midwood, K. S. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010, 672395 (2010).
Rock, K. L., Latz, E., Ontiveros, F. & Kono, H. The sterile inflammatory response. Annu. Rev. Immunol. 28, 321–342 (2010).
Foell, D., Wittkowski, H. & Roth, J. Monitoring disease activity by stool analyses: from occult blood to molecular markers of intestinal inflammation and damage. Gut 58, 859–868 (2009).
Palone, F. et al. Role of HMGB1 as a Suitable Biomarker of Subclinical Intestinal Inflammation and Mucosal Healing in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 20, 1448–1457 (2014).
Neves, A. R. et al. Overexpression of ATP-activated P2X7 receptors in the intestinal mucosa is implicated in the pathogenesis of Crohn's disease. Inflamm. Bowel Dis. 20, 444–57 (2014).
Maeda, S. et al. Essential roles of high-mobility group BOX 1 in the development of murine colitis and colitis-associated cancer. Biochem. Biophys. Res. Commun. 360, 394–400 (2007).
Dave, S. H. et al. Ethyl pyruvate decreases HMGB1 release and ameliorates murine colitis. J. Leukoc. Biol. 86, 633–643 (2009).
Schiering, C. et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513, 564–568 (2014).
Scarpa, M. et al. Interleukin-1α, an epithelial danger signal, is a potent activator of fibroblasts and reactivator of intestinal inflammation. Am. J. Pathol. 185, 1624–1637 (2015).
El Mezayen, R. et al. Endogenous signals released from necrotic cells augment inflammatory responses to bacterial endotoxin. Immunol. Lett. 111, 36–44 (2007).
Nathan, C. & Ding, A. Nonresolving inflammation. Cell 140, 871–882 (2010).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
H.S.P.d.S. and C.F. declare no competing interests.
Supplementary information
Supplementary Table 1
Genetic polymorphisms associated with Crohn's disease and implications for pathogenic mechanisms (DOC 70 kb)
Supplementary Table 2
Genetic polymorphisms associated with ulcerative colitis and implications for pathogenic mechanisms (DOC 56 kb)
Supplementary Table 3
Genetic polymorphisms associated with both Crohn's disease and ulcerative colitis and implications for pathogenic mechanisms (DOC 169 kb)
Rights and permissions
About this article
Cite this article
de Souza, H., Fiocchi, C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol 13, 13–27 (2016). https://doi.org/10.1038/nrgastro.2015.186
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2015.186
This article is cited by
-
Activation of mucosal insulin receptor exacerbates intestinal inflammation by promoting tissue resident memory T cells differentiation through EZH2
Journal of Translational Medicine (2024)
-
Hyperactivation and enhanced cytotoxicity of reduced CD8+ gamma delta T cells in the intestine of patients with Crohn’s disease correlates with disease activity
BMC Immunology (2024)
-
Increased CpG methylation at the CDH1 locus in inflamed ileal mucosa of patients with Crohn disease
Clinical Epigenetics (2024)
-
EFHD2 suppresses intestinal inflammation by blocking intestinal epithelial cell TNFR1 internalization and cell death
Nature Communications (2024)
-
Endoplasmic reticulum stress interferes with the development of type 1 regulating T cells
Inflammation Research (2024)