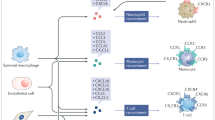
Key Points
-
There is growing evidence that heterophilic interactions between chemokines serve to fine tune leukocyte responses and have specific roles in the pathophysiology of inflammatory diseases. Such interactions are therefore potential drug targets.
-
The chemokine system consists of many components that orchestrate leukocyte trafficking. Through differential presentation of chemokines in a particular microenvironment, specific leukocyte subsets can be attracted for a tailored response during immune surveillance and inflammation.
-
The affinity of chemokines for glycosaminoglycans and their propensity to form multimeric structures is crucial for their cellular presentation. Mutant chemokines that lack these properties are inactive in vivo.
-
Platelets store and secrete various chemokines. Deposition of CC-chemokine ligand 5 (CCL5) and CXC-chemokine ligand 4 (CXCL4) onto endothelial cells by activated platelets has been implicated in atherogenic vascular inflammation.
-
Chemokines have an important role in the progression of atherosclerosis. Blockade or deletion of elements in the chemokine systems attenuates atherosclerotic disease and combined interference with multiple chemokine functions nearly abolishes atherosclerosis in mice.
-
CCL5 and CXCL4 form heteromers with greater inflammatory potential than the monomers. Disruption of this prototypical heterophilic interaction was recently shown to reduce atherosclerosis, without immunological side effects.
-
Whereas antagonism of single chemokine receptors for treating inflammatory diseases has yielded disappointing results, interference with the formation of chemokine heteromers might offer novel perspectives for specialized treatment of immune and inflammatory disorders.
Abstract
Atherosclerosis is a chronic inflammatory disease of the arterial wall that is characterized by a disturbed equilibrium of immune responses and lipid accumulation, leading to the development of plaques. The atherogenic influx of mononuclear cells is orchestrated by chemokines and their receptors. Studies using gene-deficient mice and antagonists based on peptides and small molecules have generated insight into targeting chemokine–receptor axes for treating atherosclerosis, which might complement lipid-lowering strategies and risk factor modulation. Combined inhibition of multiple chemokine axes could interfere with the contributions of chemokines to disease progression at specific cells, stages or sites. In addition, the recently characterized heterophilic interactions of chemokines might present a novel target for the treatment and prevention of inflammatory diseases such as atherosclerosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hansson, G. K. & Libby, P. The immune response in atherosclerosis: a double-edged sword. Nature Rev. Immunol. 6, 508–519 (2006).
Simionescu, M. Implications of early structural-functional changes in the endothelium for vascular disease. Arterioscler. Thromb. Vasc. Biol. 27, 266–274 (2007).
Simionescu, N., Vasile, E., Lupu, F., Popescu, G. & Simionescu, M. Prelesional events in atherogenesis. Accumulation of extracellular cholesterol-rich liposomes in the arterial intima and cardiac valves of the hyperlipidemic rabbit. Am. J. Pathol. 123, 109–125 (1986).
Zernecke, A., Shagdarsuren, E. & Weber, C. Chemokines in atherosclerosis: an update. Arterioscler. Thromb. Vasc. Biol. 28, 1897–1908 (2008).
Charo, I. F. & Taubman, M. B. Chemokines in the pathogenesis of vascular disease. Circ. Res. 95, 858–866 (2004).
Ley, K., Laudanna, C., Cybulsky, M. I. & Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature Rev. Immunol. 7, 678–689 (2007).
Weber, C., Fraemohs, L. & Dejana, E. The role of junctional adhesion molecules in vascular inflammation. Nature Rev. Immunol. 7, 467–477 (2007).
Mehrad, B., Keane, M. P. & Strieter, R. M. Chemokines as mediators of angiogenesis. Thromb. Haemost. 97, 755–762 (2007).
Jin, D. K. et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nature Med. 12, 557–567 (2006).
Kryczek, I. et al. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 65, 465–472 (2005).
Ma, Q. et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc. Natl Acad. Sci. USA 95, 9448–9453 (1998).
Tachibana, K. et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 393, 591–594 (1998).
Loetscher, P. et al. The ligands of CXC chemokine receptor 3, I-TAC, Mig, and IP10, are natural antagonists for CCR3. J. Biol. Chem. 276, 2986–2991 (2001).
Proost, P. et al. Amino-terminal truncation of chemokines by CD26/dipeptidyl-peptidase IV. Conversion of RANTES into a potent inhibitor of monocyte chemotaxis and HIV-1-infection. J. Biol. Chem. 273, 7222–7227 (1998).
Proost, P. et al. Citrullination of CXCL8 by peptidylarginine deiminase alters receptor usage, prevents proteolysis, and dampens tissue inflammation. J. Exp. Med. 205, 2085–2097 (2008).
Tacke, F. et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117, 185–194 (2007).
Combadiere, C. et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6Chi and Ly6Clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 117, 1649–1657 (2008). This study shows the potential benefits of combined chemokine blockade in the prevention of atherosclerosis.
Witt, D. P. & Lander, A. D. Differential binding of chemokines to glycosaminoglycan subpopulations. Curr. Biol. 4, 394–400 (1994).
Kuschert, G. S. et al. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry 38, 12959–12968 (1999).
de Paz, J. L. et al. Profiling heparin-chemokine interactions using synthetic tools. ACS Chem. Biol. 2, 735–744 (2007).
Weber, K. S., von Hundelshausen, P., Clark-Lewis, I., Weber, P. C. & Weber, C. Differential immobilization and hierarchical involvement of chemokines in monocyte arrest and transmigration on inflamed endothelium in shear flow. Eur. J. Immunol. 29, 700–712 (1999).
Shamri, R. et al. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nature Immunol. 6, 497–506 (2005).
Huo, Y. et al. The chemokine KC, but not monocyte chemoattractant protein-1, triggers monocyte arrest on early atherosclerotic endothelium. J. Clin. Invest. 108, 1307–1314 (2001).
Weber, C. et al. Specialized roles of the chemokine receptors CCR1 and CCR5 in the recruitment of monocytes and TH1-like/CD45RO+ T cells. Blood 97, 1144–1146 (2001).
Clore, G. M. & Gronenborn, A. M. Three-dimensional structures of alpha and beta chemokines. FASEB J. 9, 57–62 (1995).
Rajarathnam, K. et al. Neutrophil activation by monomeric interleukin-8. Science 264, 90–92 (1994).
Proudfoot, A. E. et al. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc. Natl Acad. Sci. USA 100, 1885–1890 (2003).
Fernando, H., Chin, C., Rosgen, J. & Rajarathnam, K. Dimer dissociation is essential for interleukin-8 (IL-8) binding to CXCR1 receptor. J. Biol. Chem. 279, 36175–36178 (2004).
Rajarathnam, K., Prado, G. N., Fernando, H., Clark-Lewis, I. & Navarro, J. Probing receptor binding activity of interleukin-8 dimer using a disulfide trap. Biochemistry 45, 7882–7888 (2006).
Vives, R. R., Sadir, R., Imberty, A., Rencurosi, A. & Lortat-Jacob, H. A kinetics and modeling study of RANTES(9–68) binding to heparin reveals a mechanism of cooperative oligomerization. Biochemistry 41, 14779–14789 (2002).
Hoogewerf, A. J. et al. Glycosaminoglycans mediate cell surface oligomerization of chemokines. Biochemistry 36, 13570–13578 (1997).
Martin, L. et al. Structural and functional analysis of the RANTES-glycosaminoglycans interactions. Biochemistry 40, 6303–6318 (2001).
Proudfoot, A. E. et al. The BBXB motif of RANTES is the principal site for heparin binding and controls receptor selectivity. J. Biol. Chem. 276, 10620–10626 (2001).
Baltus, T., Weber, K. S., Johnson, Z., Proudfoot, A. E. & Weber, C. Oligomerization of RANTES is required for CCR1-mediated arrest but not CCR5-mediated transmigration of leukocytes on inflamed endothelium. Blood 102, 1985–1988 (2003).
Ali, S., Palmer, A. C., Banerjee, B., Fritchley, S. J. & Kirby, J. A. Examination of the function of RANTES, MIP-1a, and MIP-1b following interaction with heparin-like glycosaminoglycans. J. Biol. Chem. 275, 11721–11727 (2000).
Campanella, G. S. et al. Oligomerization of CXCL10 is necessary for endothelial cell presentation and in vivo activity. J. Immunol. 177, 6991–6998 (2006).
Wang, L., Fuster, M., Sriramarao, P. & Esko, J. D. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nature Immunol. 6, 902–910 (2005). An interesting study demonstrating that the presence of functional glycosaminoglycans is essential for the activity of chemokines in vivo.
Middleton, J. et al. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell 91, 385–395 (1997).
Colditz, I. G., Schneider, M. A., Pruenster, M. & Rot, A. Chemokines at large: in-vivo mechanisms of their transport, presentation and clearance. Thromb. Haemost. 97, 688–693 (2007).
Pruenster, M. et al. The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nature Immunol. 10, 101–108 (2009).
Di Liberto, D. et al. Role of the chemokine decoy receptor D6 in balancing inflammation, immune activation, and antimicrobial resistance in Mycobacterium tuberculosis infection. J. Exp. Med. 205, 2075–2084 (2008).
Henn, V. et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 391, 591–594 (1998).
von Hundelshausen, P. et al. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation 103, 1772–1777 (2001).
Schober, A. et al. Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation 106, 1523–1529 (2002). The studies reported in references 43 and 44 describe the deposition of CCL5 onto the endothelium by activated platelets and its relevance for vascular inflammation.
Massberg, S. et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J. Exp. Med. 196, 887–896 (2002).
Huo, Y. et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nature Med. 9, 61–67 (2003).
Tans, G. et al. Comparison of anticoagulant and procoagulant activities of stimulated platelets and platelet-derived microparticles. Blood 77, 2641–2648 (1991).
Garcia, B. A. et al. The platelet microparticle proteome. J. Proteome Res. 4, 1516–1521 (2005).
Mause, S. F., von Hundelshausen, P., Zernecke, A., Koenen, R. R. & Weber, C. Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler. Thromb. Vasc. Biol. 25, 1512–1518 (2005).
Baltus, T. et al. Differential and additive effects of platelet-derived chemokines on monocyte arrest on inflamed endothelium under flow conditions. J. Leukoc. Biol. 78, 435–441 (2005).
Brandt, E., Ludwig, A., Petersen, F. & Flad, H. D. Platelet-derived CXC chemokines: old players in new games. Immunol. Rev. 177, 204–216 (2000).
von Hundelshausen, P. et al. Heterophilic interactions of platelet factor 4 and RANTES promote monocyte arrest on endothelium. Blood 105, 924–930 (2005).
Koenen, R. R. et al. Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Nature Med. 15, 97–103 (2009). This is the first report that shows a pathophysiological function of chemokine heteromers in vivo and offers new perspectives for therapeutic intervention.
Weber, C. & Koenen, R. R. Fine-tuning leukocyte responses: towards a chemokine 'interactome'. Trends Immunol. 27, 268–273 (2006).
Allen, S. J., Crown, S. E. & Handel, T. M. Chemokine: receptor structure, interactions, and antagonism. Annu. Rev. Immunol. 25, 787–820 (2007).
Paoletti, S. et al. A rich chemokine environment strongly enhances leukocyte migration and activities. Blood 105, 3405–3412 (2005).
Sebastiani, S., Danelon, G., Gerber, B. & Uguccioni, M. CCL22-induced responses are powerfully enhanced by synergy inducing chemokines via CCR4: evidence for the involvement of first beta-strand of chemokine. Eur. J. Immunol. 35, 746–756 (2005).
Nesmelova, I. V., Sham, Y., Gao, J. & Mayo, K. H. CXC and CC chemokines form mixed heterodimers: association free energies from molecular dynamics simulations and experimental correlations. J. Biol. Chem. 283, 24155–24166 (2008).
Broxmeyer, H. E. et al. Comparative analysis of the human macrophage inflammatory protein family of cytokines (chemokines) on proliferation of human myeloid progenitor cells. Interacting effects involving suppression, synergistic suppression, and blocking of suppression. J. Immunol. 150, 3448–3458 (1993).
Krug, A. et al. IFN-producing cells respond to CXCR3 ligands in the presence of CXCL12 and secrete inflammatory chemokines upon activation. J. Immunol. 169, 6079–6083 (2002).
Vanbervliet, B. et al. The inducible CXCR3 ligands control plasmacytoid dendritic cell responsiveness to the constitutive chemokine stromal cell-derived factor 1 (SDF-1)/CXCL12. J. Exp. Med. 198, 823–830 (2003).
Gouwy, M. et al. Synergy between coproduced CC and CXC chemokines in monocyte chemotaxis through receptor-mediated events. Mol. Pharmacol. 74, 485–495 (2008).
Kuscher, K. et al. Synergy-inducing chemokines enhance CCR2 ligand activities on monocytes. Eur. J. Immunol. 39, 1118–1128 (2009).
Guan, E., Wang, J. & Norcross, M. A. Identification of human macrophage inflammatory proteins 1a and 1b as a native secreted heterodimer. J. Biol. Chem. 276, 12404–12409 (2001).
Dudek, A. Z. et al. Platelet factor 4 promotes adhesion of hematopoietic progenitor cells and binds IL-8: novel mechanisms for modulation of hematopoiesis. Blood 101, 4687–4694 (2003). The studies in references 64 and 65 are the first to describe a heteromeric interaction between chemokines and its functional consequences, respectively.
Nesmelova, I. V. et al. Platelet factor 4 and interleukin-8 CXC chemokine heterodimer formation modulates function at the quaternary structural level. J. Biol. Chem. 280, 4948–4958 (2005).
Crown, S. E., Yu, Y., Sweeney, M. D., Leary, J. A. & Handel, T. M. Heterodimerization of CCR2 chemokines and regulation by glycosaminoglycan binding. J. Biol. Chem. 281, 25438–25446 (2006).
Jansma, A., Handel, T. M. & Hamel, D. J. Chapter 2. Homo- and hetero-oligomerization of chemokines. Methods Enzymol. 461, 31–50 (2009).
Boring, L., Gosling, J., Cleary, M. & Charo, I. F. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394, 894–897 (1998).
Gu, L. et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol. Cell 2, 275–281 (1998). References 69 and 70 are pioneering studies that revealed a role for chemokines in atherosclerosis.
Cheng, C. et al. Shear stress-induced changes in atherosclerotic plaque composition are modulated by chemokines. J. Clin. Invest. 117, 616–626 (2007).
Boisvert, W. A. et al. Up-regulated expression of the CXCR2 ligand KC/GRO-a in atherosclerotic lesions plays a central role in macrophage accumulation and lesion progression. Am. J. Pathol. 168, 1385–1395 (2006).
Lutgens, E. et al. Gene profiling in atherosclerosis reveals a key role for small inducible cytokines: validation using a novel monocyte chemoattractant protein monoclonal antibody. Circulation 111, 3443–3452 (2005).
Bursill, C. A., Channon, K. M. & Greaves, D. R. The role of chemokines in atherosclerosis: recent evidence from experimental models and population genetics. Curr. Opin. Lipidol. 15, 145–149 (2004).
Galkina, E. et al. CXCR6 promotes atherosclerosis by supporting T-cell homing, interferon-g production, and macrophage accumulation in the aortic wall. Circulation 116, 1801–1811 (2007).
Veillard, N. R. et al. Differential influence of chemokine receptors CCR2 and CXCR3 in development of atherosclerosis in vivo. Circulation 112, 870–878 (2005).
Heller, E. A. et al. Chemokine CXCL10 promotes atherogenesis by modulating the local balance of effector and regulatory T cells. Circulation 113, 2301–2312 (2006).
van Wanrooij, E. J. et al. CXCR3 antagonist NBI-74330 attenuates atherosclerotic plaque formation in LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 28, 251–257 (2008).
Ait-Oufella, H. et al. Measles virus nucleoprotein induces a regulatory immune response and reduces atherosclerosis in mice. Circulation 116, 1707–1713 (2007).
Potteaux, S. et al. Role of bone marrow-derived CC-chemokine receptor 5 in the development of atherosclerosis of low-density lipoprotein receptor knockout mice. Arterioscler. Thromb. Vasc. Biol. 26, 1858–1863 (2006).
Braunersreuther, V. et al. Ccr5 but not Ccr1 deficiency reduces development of diet-induced atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 27, 373–379 (2007).
Potteaux, S. et al. Chemokine receptor CCR1 disruption in bone marrow cells enhances atherosclerotic lesion development and inflammation in mice. Mol. Med. 11, 16–20 (2005).
Liehn, E. A. et al. Ccr1 deficiency reduces inflammatory remodelling and preserves left ventricular function after myocardial infarction. J. Cell. Mol. Med. 12, 496–506 (2008).
Zadelaar, S. et al. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler. Thromb. Vasc. Biol. 27, 1706–1721 (2007).
Peeters, W. et al. Carotid atherosclerotic plaques stabilize after stroke: insights into the natural process of atherosclerotic plaque stabilization. Arterioscler. Thromb. Vasc. Biol. 29, 128–133 (2009).
Damas, J. K. et al. Stromal cell-derived factor-1a in unstable angina: potential antiinflammatory and matrix-stabilizing effects. Circulation 106, 36–42 (2002).
Kraaijeveld, A. O. et al. CC chemokine ligand-5 (CCL5/RANTES) and CC chemokine ligand-18 (CCL18/PARC) are specific markers of refractory unstable angina pectoris and are transiently raised during severe ischemic symptoms. Circulation 116, 1931–1941 (2007).
Aukrust, P. et al. Chemokines in cardiovascular risk prediction. Thromb. Haemost. 97, 748–754 (2007).
Boger, C. A. et al. RANTES gene polymorphisms predict all-cause and cardiac mortality in type 2 diabetes mellitus hemodialysis patients. Atherosclerosis 183, 121–129 (2005).
Simeoni, E. et al. Association of RANTES G-403A gene polymorphism with increased risk of coronary arteriosclerosis. Eur. Heart J. 25, 1438–1446 (2004).
McDermott, D. H. et al. Chemokine receptor mutant CX3CR1-M280 has impaired adhesive function and correlates with protection from cardiovascular disease in humans. J. Clin. Invest. 111, 1241–1250 (2003).
Niessner, A. et al. Opposite effects of CX3CR1 receptor polymorphisms V249I and T280M on the development of acute coronary syndrome. A possible implication of fractalkine in inflammatory activation. Thromb. Haemost. 93, 949–954 (2005).
Saederup, N., Chan, L., Lira, S. A. & Charo, I. F. Fractalkine deficiency markedly reduces macrophage accumulation and atherosclerotic lesion formation in CCR2−/− mice: evidence for independent chemokine functions in atherogenesis. Circulation 117, 1642–1648 (2008).
Degryse, B. & de Virgilio, M. The nuclear protein HMGB1, a new kind of chemokine? FEBS Lett. 553, 11–17 (2003).
Morand, E. F., Leech, M. & Bernhagen, J. MIF: a new cytokine link between rheumatoid arthritis and atherosclerosis. Nature Rev. Drug Discov. 5, 399–410 (2006).
Schober, A. et al. Stabilization of atherosclerotic plaques by blockade of macrophage migration inhibitory factor after vascular injury in apolipoprotein E-deficient mice. Circulation 109, 380–385 (2004).
Burger-Kentischer, A. et al. Reduction of the aortic inflammatory response in spontaneous atherosclerosis by blockade of macrophage migration inhibitory factor (MIF). Atherosclerosis 184, 28–38 (2006).
Bernhagen, J. et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nature Med. 13, 587–596 (2007). A study that revealed that the chemokine-like function of MIF can be ascribed to its role as a dual CXCR2 and CXCR4 ligand and is therefore a promising therapeutic target.
Calandra, T. et al. MIF as a glucocorticoid-induced modulator of cytokine production. Nature 377, 68–71 (1995).
Zernecke, A. et al. SDF-1a/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ. Res. 96, 784–791 (2005).
Schwarz, M. K. & Wells, T. N. New therapeutics that modulate chemokine networks. Nature Rev. Drug Discov. 1, 347–358 (2002).
Moser, B. et al. Interleukin-8 antagonists generated by N-terminal modification. J. Biol. Chem. 268, 7125–7128 (1993).
Weber, C. et al. Structural determinants of MIF functions in CXCR2-mediated inflammatory and atherogenic leukocyte recruitment. Proc. Natl Acad. Sci. USA 105, 16278–16283 (2008).
Gong, J. H. & Clark-Lewis, I. Antagonists of monocyte chemoattractant protein 1 identified by modification of functionally critical NH2-terminal residues. J. Exp. Med. 181, 631–640 (1995).
Proudfoot, A. E. et al. Extension of recombinant human RANTES by the retention of the initiating methionine produces a potent antagonist. J. Biol. Chem. 271, 2599–2603 (1996).
Simmons, G. et al. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science 276, 276–279 (1997).
Proudfoot, A. E. et al. Amino-terminally modified RANTES analogues demonstrate differential effects on RANTES receptors. J. Biol. Chem. 274, 32478–32485 (1999).
Grone, H. J. et al. Met-RANTES reduces vascular and tubular damage during acute renal transplant rejection: blocking monocyte arrest and recruitment. FASEB J. 13, 1371–1383 (1999).
Panzer, U. et al. The chemokine receptor antagonist AOP-RANTES reduces monocyte infiltration in experimental glomerulonephritis. Kidney Int. 56, 2107–2115 (1999).
Veillard, N. R. et al. Antagonism of RANTES receptors reduces atherosclerotic plaque formation in mice. Circ. Res. 94, 253–261 (2004).
Matsukawa, A. et al. Endogenous monocyte chemoattractant protein-1 (MCP-1) protects mice in a model of acute septic peritonitis: cross-talk between MCP-1 and leukotriene B4. J. Immunol. 163, 6148–6154 (1999).
Serbina, N. V. & Pamer, E. G., Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nature Immunol. 7, 311–317 (2006).
Sorensen, L. N. & Paludan, S. R. Blocking CC chemokine receptor (CCR) 1 and CCR5 during herpes simplex virus type 2 infection in vivo impairs host defence and perturbs the cytokine response. Scand. J. Immunol. 59, 321–333 (2004).
Handel, T. M. et al. An engineered monomer of CCL2 has anti-inflammatory properties emphasizing the importance of oligomerization for chemokine activity in vivo. J. Leukoc. Biol. 84, 1101–1108 (2008).
Johnson, Z. et al. Interference with heparin binding and oligomerization creates a novel anti-inflammatory strategy targeting the chemokine system. J. Immunol. 173, 5776–5785 (2004).
Braunersreuther, V. et al. A novel RANTES antagonist prevents progression of established atherosclerotic lesions in mice. Arterioscler. Thromb. Vasc. Biol. 28, 1090–1096 (2008). This study introduces a new chemokine antagonist, which is based on modification of glycosaminoglycan binding and attenuates the progression of atherosclerosis in mice.
Potzinger, H. et al. Developing chemokine mutants with improved proteoglycan affinity and knocked-out GPCR activity as anti-inflammatory recombinant drugs. Biochem. Soc. Trans. 34, 435–437 (2006).
Schuksz, M. et al. Surfen, a small molecule antagonist of heparan sulfate. Proc. Natl Acad. Sci. USA 105, 13075–13080 (2008).
Bursill, C. A., Cash, J. L., Channon, K. M. & Greaves, D. R. Membrane-bound CC chemokine inhibitor 35K provides localized inhibition of CC chemokine activity in vitro and in vivo. J. Immunol. 177, 5567–5573 (2006).
Deruaz, M. et al. Ticks produce highly selective chemokine binding proteins with antiinflammatory activity. J. Exp. Med. 205, 2019–2031 (2008).
Martin, A. P., Canasto-Chibuque, C., Shang, L., Rollins, B. J. & Lira, S. A. The chemokine decoy receptor M3 blocks CC chemokine ligand 2 and CXC chemokine ligand 13 function in vivo. J. Immunol. 177, 7296–7302 (2006).
van Wanrooij, E. J. et al. HIV entry inhibitor TAK-779 attenuates atherogenesis in low-density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 25, 2642–2647 (2005).
Nabah, Y. N. A. et al. CXCR2 blockade impairs angiotensin II induced CC chemokine synthesis and mononuclear leukocyte infiltration. Arterioscler. Thromb. Vasc. Biol. 27, 2370–2376 (2007).
Karshovska, E., Zagorac, D., Zernecke, A., Weber, C. & Schober, A. A small molecule CXCR4 antagonist inhibits neointima formation and smooth muscle progenitor cell mobilization after arterial injury. J. Thromb. Haemost. 6, 1812–1815 (2008).
Zernecke, A. et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ. Res. 102, 209–217 (2008).
Imamura, S. et al. Discovery of a piperidine-4-carboxamide CCR5 antagonist (TAK-220) with highly potent anti-HIV-1 activity. J. Med. Chem. 49, 2784–2793 (2006).
Horuk, R. Chemokine receptor antagonists: overcoming developmental hurdles. Nature Rev. Drug Discov. 8, 23–33 (2009).
Wallis, R. S. Infectious complications of tumor necrosis factor blockade. Curr. Opin. Infect. Dis. 22, 403–409 (2009).
Lionakis, M. S. & Kontoyiannis, D. P. Glucocorticoids and invasive fungal infections. Lancet 362, 1828–1838 (2003).
Yusuf, S. et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N. Engl. J. Med. 342, 145–153 (2000).
Nissen, S. E. et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N. Engl. J. Med. 356, 1304–1316 (2007).
Opar, A. Where now for new drugs for atherosclerosis? Nature Rev. Drug Discov. 6, 334–335 (2007).
Nissen, S. E. et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 291, 1071–1080 (2004).
Ridker, P. M. et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 359, 2195–2207 (2008).
Lortat-Jacob, H., Grosdidier, A. & Imberty, A. Structural diversity of heparan sulfate binding domains in chemokines. Proc. Natl Acad. Sci. USA 99, 1229–1234 (2002).
Weber, C., Zernecke, A. & Libby, P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nature Rev. Immunol. 8, 802–815 (2008).
Ait-Oufella, H. et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nature Med. 12, 178–180 (2006).
Geissmann, F., Jung, S. & Littman, D. R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 (2003).
Swirski, F. K. et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 117, 195–205 (2007).
Swirski, F. K., Weissleder, R. & Pittet, M. J. Heterogeneous in vivo behavior of monocyte subsets in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 29, 1424–1432 (2009).
van Leeuwen, M. et al. Accumulation of myeloperoxidase-positive neutrophils in atherosclerotic lesions in LDLR−/− mice. Arterioscler. Thromb. Vasc. Biol. 28, 84–89 (2008).
Bot, I. et al. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation 115, 2516–2525 (2007).
Sun, J. et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nature Med. 13, 719–724 (2007).
Acknowledgements
The authors thank E. Lutgens and O. Soehnlein for providing useful comments. Financial support for this work was from the Interdisciplinary Center for Clinical Research 'Biomat' within the Medical Faculty of RWTH Aachen University (T5 and K1) and the Deutsche Forschungsgemeinschaft (DFG-FOR809).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
R.R.K. and C.W. are shareholders of Carolus Therapeutics Inc. (San Diego, California, USA).
Related links
Glossary
- Atherosclerosis
-
A chronic inflammation of the mid-sized and larger arteries of the body, characterized by lipid-rich lesions with a necrotic core that is separated from the blood by a layer of smooth muscle cells and matrix proteins.
- Hyperlipidaemia
-
The prolonged presence of abnormally high levels of circulating lipoproteins in the bloodstream. It is an established risk factor for cardiovascular disease.
- Thrombosis
-
The pathological formation of a clot in a blood vessel that obstructs the blood flow to the downstream tissue. A hazardous complication of thrombosis is embolization, in which clot fragments are released and occlude distal vessels such as those in the lungs or brain.
- Myocardial infarction
-
The cessation of blood supply to the heart muscle that may lead to tissue damage, scar formation and cardiac insufficiency. It is generally caused by thrombotic occlusion of the coronary arteries.
- Extravasation
-
The migration of leukocytes from the bloodstream to the exterior of the blood vessel. It is also referred to as transendothelial migration or transmigration.
- Chemorheotaxis
-
Chemokine-triggered migration of leukocytes under flow conditions.
- Integrin
-
A heterodimeric transmembrane protein complex consisting of an α and a β subunit, which mediate cell–cell or cell–matrix interactions and are essential for processes such as cell trafficking or attachment.
- Cell adhesion molecule
-
A class of molecules, such as integrins, that are specialized in mediating cell adhesion to other cells or to the surrounding environment.
- Glycosaminoglycans
-
Long linear polysaccharides that are often linked to the cell membrane by a protein core. They are highly negatively charged owing the presence of carboxylic and sulphonic acid side groups on the saccharide building blocks.
- Shear flow conditions
-
Conditions of non-turbulent blood flow in veins and arteries under which the viscosity and flow rate of the blood impose a physiologically relevant force upon the vessel wall.
- CD45RO+ memory T cell
-
A T cell subtype that is important for immunological memory. Memory T cells express the CD45RO isotype on their cell surface, which can be used as a marker for their identification.
- Nuclear magnetic resonance
-
A type of spectroscopy that measures the behaviour of magnetic nuclei (for example, protons) in strong, rapidly pulsing magnetic fields. It can be used to determine the solution structure of smaller-sized proteins.
- Oligomerization
-
The assembly of single protein units (monomers) into higher-order structures (multimers or oligomers) that contain a finite number of monomers.
- Neointima formation
-
The pathological inward growth of a blood vessel wall in response to injury that is associated with accumulation and proliferation of smooth muscle cells. Neointimal hyperplasia that leads to restenosis is a complication of angioplastic interventions.
- LY6Chi monocytes
-
One of two subsets of monocytes in mice, which express high levels of lymphocyte antigen 6C (LY6C) and are considered to have inflammatory properties.
- LY6Clow monocytes
-
One of two subsets of monocytes in mice, which express low levels of lymphocyte antigen 6C (LY6C). They are precursors of macrophages and dendritic cells in healthy tissues.
- Pseudo-(E)LR motif
-
Two non-adjacent amino acid residues (Arg11 and Asp44) that are present on neighbouring loops of macrophage migration inhibitory factor and feature identical parallel spacing. The result is a mimic of the ELR motif that characterizes ligands of CXC-chemokine receptor 2.
Rights and permissions
About this article
Cite this article
Koenen, R., Weber, C. Therapeutic targeting of chemokine interactions in atherosclerosis. Nat Rev Drug Discov 9, 141–153 (2010). https://doi.org/10.1038/nrd3048
Issue Date:
DOI: https://doi.org/10.1038/nrd3048
This article is cited by
-
MK2206 attenuates atherosclerosis by inhibiting lipid accumulation, cell migration, proliferation, and inflammation
Acta Pharmacologica Sinica (2022)
-
Targeting chemokines for acute lymphoblastic leukemia therapy
Journal of Hematology & Oncology (2021)
-
A potential novel therapeutic target in diabetic retinopathy: a chemokine receptor (CCR2/CCR5) inhibitor reduces retinal vascular leakage in an animal model
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)
-
Viromers as carriers for mRNA-mediated expression of therapeutic molecules under inflammatory conditions
Scientific Reports (2020)
-
Systematic analysis of lncRNA expression profiles and atherosclerosis-associated lncRNA-mRNA network revealing functional lncRNAs in carotid atherosclerotic rabbit models
Functional & Integrative Genomics (2020)