Key Points
-
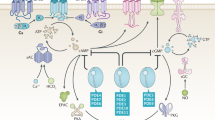
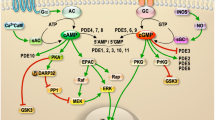
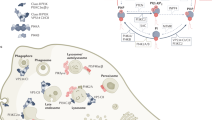
Cyclic AMP and cyclic GMP are crucial signalling molecules that allow the seamless communication of extracellular signals from diverse membrane-bound transduction systems to tertiary messenger and effector systems within a single cell.
-
Phosphodiesterases (PDEs) are a class of enzymes that regulate cAMP and cGMP concentrations throughout the body.
-
Inhibitors of PDEs (for example, sildenafil, which targets PDE5) have become an important class of drugs, with applications in erectile dysfunction and pulmonary hypertension.
-
The PDE gene superfamily consists of 11 PDE families that are made up of 21 genes that are, in turn, transcribed into more than 50 functionally unique enzymes by alternate splicing.
-
Most PDEs are expressed in the CNS, making this gene superfamily a particularly attractive source of new targets for the treatment of both psychiatric and neurodegenerative disorders.
-
A gene-family drug discovery approach has been used with the PDEs, which enables efficient reagent production, assay development and lead discovery.
Abstract
The therapeutic and commercial success of phosphodiesterase 5 inhibitors such as Viagra, Levitra and Cialis has sparked renewed interest in the phosphodiesterases as drug discovery targets. Virtually all the phosphodiesterases are expressed in the CNS, making this gene family a particularly attractive source of new targets for the treatment of psychiatric and neurodegenerative disorders. Significantly, all neurons express multiple phosphodiesterases, which differ in cyclic nucleotide specificity, affinity, regulatory control and subcellular compartmentalization. Therefore, phosphodiesterase inhibition represents a mechanism through which it could be possible to precisely modulate neuronal activity. In this article, we review the current state of the art in the burgeoning field of phosphodiesterase pharmacology in the CNS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sutherland, E. W. Studies on the mechanism of hormone action. Science 177, 401–408 (1972).
Houslay, M. D. Compartmentalization of cyclic AMP phosphodiesterases, signaling 'crosstalk', desensitization and the phosphorylation of Gi-2 add cell specific personalization to the control of the levels of the second messenger cyclic AMP. Advan. Enzyme Regul. 35, 303–338 (1995).
Houslay, M. D. & Adams, D. R. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem. J. 370, 1–18 (2003). Comprehensive review of the physiology of PDE4. The principles regarding the structure and compartmentalization of PDE4 apply widely to the other phosphodiesterases.
Baillie, G. S. & Houslay, M. D. Arrestin times for compartmentalised cAMP signaling and phosphodiesterase-4 enzymes. Curr. Opin. Cell Biol. 17, 129–134 (2005).
Finn, J. T., Grunwald, M. E. & Yau, K. W. Cyclic nucleotide-gated ion channels: an extended family with diverse functions. Annu. Rev. Physiol. 58, 395–426 (1996).
Beltman, J., Sonnenburg, W. K. & Beavo, J. A. The role of protein phosphorylation in the regulation of cyclic nucleotide phosphodiesterases. Mol. Cell. Biochem. 127, 239–253 (1993).
Kawaski, H. et al. A family of cAMP-binding proteins that directly activate Rap1. Science 282, 2275–2279 (1998).
Bos, J. L. EPAC: a new cAMP target and new avenues in cAMP research. Nature Rev. Mol. Cell Biol. 4, 733–738 (2003).
Colledge, M. & Scott, J. D. AKAPs: from structure to function. Trends Cell Biol. 9, 216–221 (1999).
Lugnier, C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol. Ther. 109, 366–398 (2006). A recent comprehensive review of the PDE superfamily.
Beavo, J. A., Conti, M. & Hasslip, R. J. Multiple cyclic nucleotide phosphodiesterases. Mol. Pharmacol. 46, 399–405 (1994).
Houslay, M. D. & Milligan, G. Tailoring cAMP-signalling responses through isoform multiplicity. Trends Biochem. Sci. 22, 217–224 (1997).
Sasaki, T., Kotera, J. & Oomori, K. Novel alternative splice variants of rat phosphodiesterase 7B showing unique tissue-specific expression and phosphorylation. Biochem. J. 361, 211–220 (2002).
Xie, Z. et al. Cellular and subcellular localization of PDE10A, a striatal-specific phosphodiesterase. Neuroscience 139, 597–607 (2006).
Noyama, K. & Maekawa, S. Localization of cyclic nucleotide phosphodiesterase 2 in the brain-derived Triton-insoluble low-density fraction (raft). Neurosci. Res. 45, 141–148 (2003).
Cherry, J. A. & Davis, R. L. Cyclic AMP phospho-diesterases are localized in regions of the mouse brain associated with reinforcement, movement, and affect. J. Comp. Neurol. 407, 287–301 (1999).
Houslay, M. D., Schafer, P. & Zhang, K. Y. Phosphodiesterase-4 as a therapeutic target. Drug Discov. Today 10, 1503–1519 (2005).
Dudai, Y., Jan, Y. N., Byers, D., Quinn, W. G. & Benzer, S. dunce, a mutant of Drosophila deficient in learning. Proc. Natl Acad. Sci. USA 73, 1684–1688 (1976).
Rose, G. M., Hopper, A., De Vivo, M. & Tehim, A. Phosphodiesterase inhibitors for cognitive enhancement. Curr. Pharm. Des. 11, 3329–3334 (2005).
Barad, M., Bourtchouladze, R., Winder, D. G., Golan, D. G. & Kandel, E. R. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc. Natl Acad. Sci. USA 95, 15020–15025 (1998).
Monti, B., Berteotti, C. & Contestabile, A. Subchronic Rolipram delivery activates hippocampal CREB and Arc, enhances retention and slows down extinction of conditioned fear. Neuropsychopharmacology 31, 278–286 (2006).
O'Donnell, J. M. & Zhang, H. T. Antidepressant effects of inhibitors of cAMP phosphodiesterase (PDE4). Trends Pharmacol. Sci. 25, 158–163 (2004).
Zeller, E. Results of a phase II study of the antidepressant effect of rolipram. Pharmacopsychiatry 17, 188–190 (1984).
Monnet, F. P. Transcription and neurotrophic factors in affective disorders: new trends in understanding the action of antidepressant drugs. Curr. Psychiatry Rev. 1, 313–317 (2005).
Nakagawa, S. et al. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J. Neurosci. 22, 3673–3682 (2002).
Nibuya, M., Nestler, E. J. & Duman, R. S. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J. Neurosci. 16, 2365–2372 (1996).
Maxwell, C. R., Kanes, S. J., Abel, T. & Siegel, S. J. Phosphodiesterase inhibitors: a novel mechanism for receptor-independent antipsychotic medications. Neuroscience 129, 101–107 (2004).
Millar, J. K. et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science 310, 1128–1129 (2005).
Yamashita, N., Hayashi, A., Baba, J. & Sawa, A. Rolipram, a phosphodiesterase-4-selective inhibitor, promotes the survival of cultured rat dopaminergic neurons. Jpn. J. Pharmacol. 75, 155–159 (1997).
Akaike, N., Furukawa, K. & Kogure, K. Rolipram enhances the development of voltage-dependent Ca2+ current and serotonin-induced current in rat pheochromocytoma cells. Brain Res. 620, 58–63 (1993).
Pearse, D. D. et al. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nature Med. 10, 610–616 (2004).
Gao, Y., Nikulina, E., Mellado, W. & Filbin, M. T. Neurotrophins elevate cAMP to reach a threshold required to overcome inhibition by MAG through extracellular signal-regulated kinase-dependent inhibition of phosphodiesterase. J. Neurosci. 23, 11770–11777 (2003).
Gong, B. et al. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J. Clin. Invest. 114, 1624–1634 (2004).
Conti, M. et al. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J. Biol. Chem. 278, 5493–5496 (2003).
Robichaud, A. et al. Deletion of phosphodiesterase 4D in mice shortens 2-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J. Clin. Invest. 110, 1045–1052 (2002).
Kalgutkar, A. S., Choo, E., Taylor, T. J. & Marfat, A. Disposition of CP-671,305, a selective phosphodiesterase 4 inhibitor in preclinical species. Xenobiotica 34, 755–770 (2004).
Ahmed, T. & Frey, J. U. Expression of the specific type IV phosphodiesterase gene PDE4B3 during different phases of LTP in single hippocampal slices of rats in vitro. Neuroscience 117, 627–638 (2003).
Miro, X., Perez-Torres, S., Palacios, J. M., Puigdomenech, P. & Mengod, G. Differential distribution of cAMP-specific phosphodiesterase 7A mRNA in rat brain and peripheral organs. Synapse 40, 201–214 (2001).
Sasaki, T., Kotera, J. & Omori, K. Novel alternative splice variants of rat phosphodiesterase 7B showing unique tissue-specific expression and phosphorylation. Biochem. J. 361, 211–220 (2002).
Reyes-Irisarri, E., Perez, S. & Mengod, G. Neuronal expression of cAMP-specific phosphodiesterase 7B in the rat brain. Neuroscience 132, 1173–1185 (2005).
Sasaki, T., Kotera, J. & Omori, K. Transcriptional activation of phosphodiesterase 7B1 by dopamine D1 receptor stimulation through the cyclic AMP/cyclic AMP-dependent protein kinase/cyclic AMP-response element binding protein pathway in primary striatal neurons. J. Neurochem. 89, 474–483 (2004).
Perez-Torres, S. et al. Alteration of phosphodiesterase type 7 and 8 isozyme mRNA expression in Alzheimer's disease brains examined by in situ hybridization. Exp. Neurol. 182, 322–334 (2003).
Sasaki, T., Yuasa, K. & Omori, K. Identification of human PDE7B, a cAMP specific phosphodiesterase. Biochem. Biophys. Res. Comm. 271, 575–583 (2000).
Gardner, C., Robas, N., Cawkill, D. & Fidock, M. Cloning and characterization of the human and mouse PDE7B, a novel cAMP-specific cyclic nucleotide phosphodiesterase. Biochem. Biophys. Res. Comm. 272, 186–192 (2000).
Barnes, M. J. et al. Synthesis and structure–activity relationships of guanine analogues as phosphodiesterase 7 (PDE7) inhibitors. Bioorg. Med. Chem. Lett. 11, 1081–1083 (2001).
Pitts, W. J. et al. Identification of purine inhibitors of phosphodiesterase 7 (PDE7). Bioorg. Med. Chem. Lett. 14, 2955–2958 (2004).
Vergne, F. et al. Discovery of thiadiazoles as a novel structural class of potent and selective PDE7 inhibitors. Part 1: design, synthesis and structure–activity relationship studies. Bioorg. Med. Chem. Lett. 14, 4607–4613 (2004).
Kobayashi, T. et al. Molecular comparison of rat cyclic nucleotide phosphodiesterase 8 family: unique expression of PDE8B in rat brain. Gene 319, 221–231 (2003).
Prickaerts, J. et al. Effects of two selective phosphodiesterase type 5 inhibitors, sildenafil and vardenafil, on object recognition memory and hippocampal cyclic GMP levels in the rat. Neuroscience 113, 351–361 (2002).
Prickaerts, J. et al. Phosphodiesterase type 5 inhibition improves early memory consolidation of object information. Neurochem. Int. 45, 915–928 (2004).
Devan, B. D. et al. Phosphodiesterase inhibition by sildenafil citrate attenuates the learning impairment induced by blockade of cholinergic muscarinic receptors in rats. Pharmacol. Biochem. Behav. 79, 691–699 (2004).
Kotera, J. et al. Expression of rat cGMP-binding cGMP-specific phosphodiesterase mRNA in Purkinje cell layers during postnatal neuronal development. Eur. J. Biochem. 249, 434–442 (1997).
Kotera, J., Fujishige, K. & Omori, K. Immunohistochemical localization of cGMP-binding cGMP-specific phosphodiesterase (PDE5) in rat tissues. J. Histochem. Cytochem. 48, 685–693 (2000).
Rapoport, M., van Reekum, R. & Mayberg, H. The role of the cerebellum in cognition and behavior. J. Neuropsychiatry Clin. Neurosci. 12, 193–198 (2000).
Hartell, N. A. Inhibition of cGMP breakdown promotes the induction of cerebellar long-term depression. J. Neurosci. 16, 2881–2890 (1996).
van Staveren, W. C. et al. Cloning and localization of the cGMP-specific phosphodiesterase type 9 in the rat brain. J. Neurocytol. 31, 729–741 (2002).
Schultheiss, D. et al. Central effects of sildenafil (Viagra) on auditory selective attention and verbal recognition memory in humans: a study with event-related brain potentials. World J. Urol. 19, 46–50 (2001).
Andreeva, S. G., Dikkes, P., Epstein, P. M. & Rosenberg, P. A. Expression of cGMP-specific phosphodiesterase 9A mRNA in the rat brain. J. Neurosci. 21, 9068–9076 (2001).
Guipponi, M. et al. Identification and characterization of a novel cyclic nucleotide phosphodiesterase gene (PDE9A) that maps to 21q22.3: alternative splicing of mRNA transcripts, genomic structure and sequence. Hum. Genet. 103, 386–392 (1998).
Straub, R. E. et al. A possible vulnerability locus for bipolar affective disorder on chromosome 21q22.3. Nature Genet. 8, 291–296 (1994).
Detera-Wadleigh, S. D. et al. Affected-sib-pair analyses reveal support of prior evidence for a susceptibility locus for bipolar disorder, on 21q. Am. J. Hum. Genet. 58, 1279–1285 (1996).
Gurling, H. Chromosome 21 workshop. Psychiatr. Genet. 8, 109–113 (1998).
Wunder, F. et al. Characterization of the first potent and selective PDE9 inhibitor using a cGMP reporter cell line. Mol. Pharm. 68, 1775–1781 (2005).
Sonnenburg, W. K., Mullaney, P. J. & Beavo, J. A. Molecular cloning of a cyclic GMP-stimulated cyclic nucleotide phosphodiesterase cDNA. Identification and distribution of isozyme variants. J. Biol. Chem. 266, 17655–17661 (1991).
Martins, T. J., Mumby, M. C. & Beavo, J. A. Purification and characterization of a cyclic nucleotide GMP-stimulated cyclic nucleotide phosphodiesterase from bovine tissues. J. Biol. Chem. 257, 1973–1979 (1982).
Repaske, D. R., Corbin, J. G., Conti, M. & Goy, M. F. A cyclic GMP-stimulated cyclic nucleotide phosphodiesterase gene is highly expressed in the limbic system of the rat brain. Neuroscience 56, 673–686 (1993).
Juilfs, D. M. et al. A subset of olfactory neurons that selectively express cGMP-stimulated phosphodiesterase (PDE2) and guanylyl cyclase-D define a unique olfactory transduction pathway. Proc. Natl Acad. Sci. USA 94, 3388–3395 (1997).
Meyer, M. R., Angele, A., Kremme, E., Kaupp, U. B. & Muller, F. A cGMP-signaling pathway in a subset of olfactory sensory neurons. Proc. Natl Acad. Sci. USA 97, 10595–10600 (2000).
Coleman, R. W. Platelet cyclic adenosine monophosphate phosphodiesterases: targets for regulating platelet-related thrombosis. Semin. Thromb. Hemost. 30, 451–460 (2004).
Velardez, M. O. et al. Role of phosphodiesterase and protein kinase G on nitric oxide-induced inhibition of prolactin release from the rat anterior pituitary. Eur. J. Endocrinol. 143, 279–284 (2000).
Suvarna, N. U. & O'Donnell, J. M. Hydrolysis of N-methyl-D-aspartate receptor stimulated cAMP and cGMP by PDE4 and PDE2 phosphodiesterases in primary neuronal cultures of rat cerebral cortex and hippocampus. J. Pharmacol. Exp. Ther. 302, 249–256 (2002).
Boess, F. G. et al. Inhibition of phosphodiesterase 2 increases neuronal cGMP, synaptic plasticity and memory performance. Neuropharmacology 47, 1081–1092 (2004).
Strick, C. A., Schmidt, C. J. & Menniti, F. S. in Phosphodiesterases in Health and Disease (eds Francis, S., Houslay, M. & Beavo, J.) (CRC, London 2006).
Soderling, S. H., Bayuga, S. J. & Beavo, J. A. Isolation and characterization of a dual-substrate phosphodiesterase gene family: PDE10A. Proc. Natl Acad. Sci. USA 96, 7071–7076 (1999).
Loughney, K. et al. Isolation and characterization of PDE10A, a novel human 3′,5'-cyclic nucleotide phosphodiesterase. Gene 234, 109–117 (1999).
Fujishige, K., Kotera, J. & Omori, K. Striatum- and testis-specific phosphodiesterase PDE10A isolation and characterization of a rat PDE10A. Eur. J. Biochem. 266, 1118–1127 (1999).
Seeger, T. F. et al. Immunohistochemical localization of PDE10A in the rat brain. Brain Res. 985, 113–126 (2003).
Graybiel, A. M. The basal ganglia. Curr. Biol. 10, R509–R511 (2000).
Siuciak, J. A. et al. Genetic deletion of the striatum-enriched phosphodiesterase PDE10A: evidence for altered striatal function. Neuropharmacology 6 June 2006 [epub ahead of print].
Siuciak, J. A. et al. Inhibition of the striatum-enriched phosphodiesterase PDE10A: a novel approach to the treatment of psychosis. Neuropharmacology 14 June 2006 [epub ahead of print].
O'Connor, V. et al. Differential amplification of intron-containing transcripts reveals long term potentiation-associated up-regulation of specific Pde10A phosphodiesterase splice variants. J. Biol. Chem. 279, 15841–15849 (2004).
Jeon, Y. H. et al. Phosphodiesterase: overview of protein structures, potential therapeutic applications and recent progress in drug development. Cell. Mol. Life Sci. 62, 1198–1220 (2005).
Wang, H., Liu, Y., Chen, Y., Robinson, H. & Ke, H. Multiple elements jointly determine inhibitor selectivity of cyclic nucleotide phosphodiesterases 4 and 7. J. Biol. Chem. 280, 30949–30955 (2005).
Ecker, G. F. & Noe, C. R. In silico prediction models for blood–brain barrier permeation. Curr. Med. Chem. 11, 1617–1628 (2004).
Fromm, M. F. Importance of P-glycoprotein at blood–tissue barriers. Trends Pharmacol. Sci. 25, 423–429 (2004).
Noridner, U. & Haeberlein, M. Computational approaches to the prediction of blood–brain distribution. Adv. Drug Deliv. Rev. 54, 291–313 (2002).
Clark, D. E. Rapid calculation of polar molecular surface area and its application to predict transport phenomena. 2. Prediction of blood–brain barrier penetration. J. Pharm. Sci. 88, 815–821 (1999).
Card, G. L. et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure 12, 2233–2247 (2004). In-depth analysis of the structure of the PDEs derived from X-ray crystallography and how this information informs the drug discovery process.
Seelig, A. A general pattern for substrate recognition by P-glycoprotein. Eur. J. Biochem. 251, 252–261 (1998).
Pajeva, I. K. & Wiese, M. Pharmacophore model of drugs involved in P-glycoprotein multidrug resistance: explanation of structural variety (hypothesis). J. Med. Chem. 45, 5671–5686 (2002).
Rotella, D. P. Phosphodiesterase 5 inhibitors: current status and potential applications. Nature Rev. Drug Discov. 1, 674–682 (2002).
Padma-Nathan, H. Efficacy and tolerability of Tadalafil, a novel phosphodiesterase 5 inhibitor, in treatment of erectile dysfunction. Am. J. Cardiol. 92 (Suppl.) 19M–25M (2003).
Barnes, P. J. New drugs for asthma. Nature Rev. Drug Discov. 3, 831–844 (2004).
Dyke, H. J. & Montana, J. G. Update of the therapeutic potential of PDE4 inhibitors. Expert Opin. Investig. Drugs 11, 1–13 (2002).
Lipworth, B. J. Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary disease. Lancet 365, 167–175 (2005).
Snyder, P. B., Esselstyn, J. M., Loughney, K., Wolda, S. L. & Florio, V. A. The role of cyclic nucleotide phosphodiesterases in the regulation of adipocyte lipolysis. J. Lipid Res. 46, 494–503 (2005).
Chambers, R. J. et al. A new chemical tool for exploring the physiological function of the PDE2 isozyme. Bioorg. Med. Chem. Lett. 16, 307–310 (2006).
Shakur, Y. et al. Membrane localization of cyclic nucleotide phosphodiesterase 3 (PDE3). J. Biol. Chem. 275, 38749–38761 (2000).
Reinhardt, R. R. & Bondy, C. A. Differential cellular pattern of gene expression for two distinct cGMP-inhibited cyclic nucleotide phosphodiesterases in developing and mature rat brain. Neuroscience 72, 567–578 (1996).
Lin, C. S. Tissue expression, distribution and regulation of PDE5. Int. J. Impot. Res. 16, S8–S10 (2004).
Acknowledgements
We thank C. A. Strick and L. James for providing data on PDE expression in mouse striatum, C.A.S, M. O'Donnell and D. Stephenson for images of PDE immunohistochemistry and J. Harms for cGMP data. We also thank X. Hou and R. Chambers for helpful discussions on targeting PDE inhibitors to the CNS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors are employees of Pfzier Inc, which developed and markets sildenafil.
Glossary
- Subcellular compartmentalization
-
A specific environment or location within a cell structure that is separate and distinct from other components of the cell.
- Long-term potentiation
-
(LTP). The prolonged strengthening of synaptic communication, which is induced by patterned input and is thought to be involved in learning and memory formation.
- Auditory evoked potentials
-
An electrical response recorded in different brain regions to a discrete auditory stimulus that represents the ensemble firing of different neuronal populations.
- Forced swim test and tail suspension test
-
Behavioural tests in rodents in which the latency to, and/or duration of, immobility is used as an index of behavioural despair. Antidepressants increase latency to, and decrease duration of, immobility.
- Single nucleotide polymorphism
-
(SNP). A location in a DNA sequence at which different nucleotides are present across a population. Differences in a single nucleotide could change the protein sequence or regulation of gene expression. This might be associated with a difference in susceptibility to a disease, or could have no consequence.
- Synaptic plasticity
-
A change in the functional properties of a synapse as a result of use.
- Spontaneous alternation
-
A behavioural test in which rodents will spontaneously alternate visits to different locations in a maze, reflecting a natural foraging strategy. This requires animals to remember their previous response, and is therefore a measure of memory function.
- Conditioned avoidance response
-
A behavioural test in which animals are trained to avoid a shock by responding to a signal stimulus. All clinically useful antipsychotic agents inhibit this response.
- Pharmacophore
-
The ensemble of steric and electronic features that is necessary to ensure optimal interactions with a specific biological target structure and to trigger (or to block) its biological response.
Rights and permissions
About this article
Cite this article
Menniti, F., Faraci, W. & Schmidt, C. Phosphodiesterases in the CNS: targets for drug development. Nat Rev Drug Discov 5, 660–670 (2006). https://doi.org/10.1038/nrd2058
Issue Date:
DOI: https://doi.org/10.1038/nrd2058
This article is cited by
-
Neuroprotective effects of vinpocetine, as a phosphodiesterase 1 inhibitor, on long-term potentiation in a rat model of Alzheimer’s disease
BMC Neuroscience (2023)
-
Selectivity mechanism of phosphodiesterase isoform inhibitor through in silico investigations
Journal of Molecular Modeling (2022)
-
How do phosphodiesterase-5 inhibitors affect cancer? A focus on glioblastoma multiforme
Pharmacological Reports (2022)
-
Molecular docking and receptor-based QASR studies on pyrimidine derivatives as potential phosphodiesterase 10A inhibitors
Structural Chemistry (2019)
-
Cyclic nucleotide phosphodiesterases: potential therapeutic targets for alcohol use disorder
Psychopharmacology (2018)