Key Points
-
One way to maximize the effect and minimize the side effects of cancer therapeutics is the targeting of drugs to the tumor by using the specificity of antibodies.
-
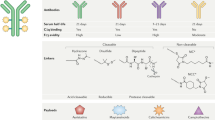
All different kinds of drug have been targeted to the tumor by this approach. In this review the targeting of chemotherapeutics, toxins, cytokines, immunomodulatory antibody fragments and siRNA to tumours is critically discussed. Of the discussed drugs, only Mylotarg, a chemotherapeutic drug conjugated to an antibody, has benn approved by the FDA so far.
-
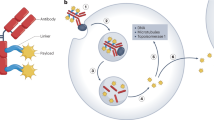
In tumour-cells not expressing antigens that can be used for targeting, antibody dependent enzyme prodrug therapy (ADEPT) can be used. ADEPT is a targeted therapy in which a weakly toxic prodrug is selectively activated into a toxic agent at the tumour site by an enzyme, which has been targeted to the tumour by a tumour-specific antibody.
-
Immunotoxins – toxins conjugated to an antibody directed to a tumour – exert strong tumour-specific cytotoxicity. However they have the disadvantage of being highly immunogenic, therefore immunotoxins consisting of human toxins are being developed.
-
Another class of antibody-targeted agent is the immunocytokines. These fusion proteins comprising an antibody and a cytokine generally exert their anti-tumor effect by enhancing and/or activating immune competent cells.
-
Finally, bispecific antibody-fragments or 'diabodies' can be used to enhance the natural immune responses to tumours or apoptosis at the site of the tumor. Several of these agents are currently being evaluated in Phase I trials.
-
In addition to being effective cancer therapeutics on their own, monoclonal antibodies look set to improve the selectivity of other types of anticancer agent if issues with immunogenicity, selectivity and tumour penetration can be addressed.
Abstract
Treatment of cancer is a double-edged sword: it should be as aggressive as possible to completely destroy the tumour, but it is precisely this aggressiveness which often causes severe side effects — a reason why some promising therapeutics can not be applied systemically. In addition, therapeutics such as cytokines that physiologically function in a para- or autocrine fashion require a locally enhanced level to exert their effect appropriately. An elegant way to accumulate therapeutic agents at the tumour site is their conjugation/fusion to tumour-specific antibodies. Here, we discuss recent preclinical and clinical data for antibody–drug conjugates and fusion proteins with a special focus on drug components that exert their antitumour effects through normal biological processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Papac, R. J. Origins of cancer therapy. Yale J. Biol. Med. 74, 391–398 (2001).
Dunn, F. B. National Cancer Act: Leaders Reflect on 30 Years of Progress. J. Natl Cancer Inst. 94, 8–9 (2002).
Schwartz, R. S. Paul Ehrlich's magic bullets. N. Engl. J. Med. 350, 1079–1080 (2004).
Köhler, G. & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. 1975. Biotechnology 24, 524–526 (1992).
Yelton, D. E. & Scharff, M. D. Monoclonal antibodies: a powerful new tool in biology and medicine. Annu. Rev. Biochem. 50, 657–680 (1981).
Riechmann, L., Clark, M., Waldmann, H. & Winter, G. Reshaping human antibodies for therapy. Nature 332, 323–327 (1988).
Liu, A. Y. et al. Chimeric mouse-human IgG1 antibody that can mediate lysis of cancer cells. Proc. Natl Acad. Sci. USA 84, 3439–3443 (1987).
Grillo-Lopez, A. J., Hedrick, E., Rashford, M. & Benyunes, M. Rituximab: ongoing and future clinical development. Semin. Oncol. 29, 105–112 (2002).
Breedveld, F. C. Therapeutic monoclonal antibodies. Lancet 355, 735–740 (2000).
Adams, G. P. & Weiner, L. M. Monoclonal antibody therapy of cancer. Nature Biotechnol. 23, 1147–1157 (2005).
Milenic, D. E., Brady, E. D. & Brechbiel, M. W. Antibody-targeted radiation cancer therapy. Nature Rev. Drug Discov. 3, 488–499 (2004). An in depth review that describes the targeting of radionucleotides to tumors by antibodies.
Johnson, J. R. et al. A vindesine–anti-CEA conjugate cytotoxic for human cancer cells in vitro. Br. J. Cancer 44, 472–475 (1981).
Garnett, M. C. Targeted drug conjugates: principles and progress. Adv. Drug Deliv. Rev. 53, 171–216 (2001).
Maxfield, F. R. & McGraw, T. E. Endocytic recycling. Nature Rev. Mol. Cell Biol. 5, 121–132 (2004).
Guillemard, V. & Uri, S. H. Prodrug chemotherapeutics bypass p-glycoprotein resistance and kill tumors in vivo with high efficacy and target-dependent selectivity. Oncogene 23, 3613–3621 (2004).
Shen, W. C., Ballou, B., Ryser, H. J. & Hakala, T. R. Targeting, internalization, and cytotoxicity of methotrexate-monoclonal anti-stage-specific embryonic antigen-1 antibody conjugates in cultured F-9 teratocarcinoma cells. Cancer Res. 46, 3912–3916 (1986).
Johnson, D. A. & Laguzza, B. C. Antitumor xenograft activity with a conjugate of a Vinca derivative and the squamous carcinoma-reactive monoclonal antibody PF1/D. Cancer Res. 47, 3118–3122 (1987).
Dillman, R. O., Johnson, D. E., Shawler, D. L. & Koziol, J. A. Superiority of an acid-labile daunorubicin-monoclonal antibody immunoconjugate compared to free drug. Cancer Res. 48, 6097–6102 (1988).
Doronina, S. O. et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nature Biotechnol. 21, 778–784 (2003).
Jaracz, S., Chen, J., Kuznetsova, L. V. & Ojima, I. Recent advances in tumor-targeting anticancer drug conjugates. Bioorg. Med. Chem. (2005).
Deguchi, T., Chu, T. M., Leong, S. S., Horoszewicz, J. S. & Lee, C. L. Effect of methotrexate-monoclonal anti-prostatic acid phosphatase antibody conjugate on human prostate tumor. Cancer Res. 46, 3751–3755 (1986).
Allen, T. M. Ligand-targeted therapeutics in anticancer therapy. Nature Rev. Cancer 2, 750–763 (2002).
Hamblett, K. J. et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin. Cancer Res. 10, 7063–7070 (2004).
Petersen, B. H., DeHerdt, S. V., Schneck, D. W. & Bumol, T. F. The human immune response to KS1/4-desacetylvinblastine (LY256787) and KS1/4-desacetylvinblastine hydrazide (LY203728) in single and multiple dose clinical studies. Cancer Res. 51, 2286–2290 (1991).
Carter, P. Improving the efficacy of antibody-based cancer therapies. Nature Rev. Cancer 1, 118–129 (2001).
Sievers, E. L. et al. Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: a phase I study of an anti-CD33 calicheamicin immunoconjugate. Blood 93, 3678–3684 (1999).
Smith, S. V. Technology evaluation: cantuzumab mertansine, ImmunoGen. Curr. Opin. Mol. Ther. 6, 666–674 (2004).
Bross, P. F. et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin. Cancer Res. 7, 1490–1496 (2001). A description of the preclinical and clinical data for Mylotarg and the regulatory review leading to approval.
Sievers, E. L. & Linenberger, M. Mylotarg: antibody-targeted chemotherapy comes of age. Curr. Opin. Oncol. 13, 522–527 (2001).
Nabhan, C. et al. Phase II pilot trial of gemtuzumab ozogamicin (GO) as first line therapy in acute myeloid leukemia patients age 65 or older. Leuk. Res. 29, 53–57 (2005).
Tallman, M. S., Gilliland, D. G. & Rowe, J. M. Drug therapy of acute myeloid leukemia. Blood (2005).
Arceci, R. J. et al. Safety and efficacy of gemtuzumab ozogamicin (Mylotarg(R)) in pediatric patients with advanced CD33-positive acute myeloid leukemia. Blood (2005).
Sanderson, R. J. et al. In vivo drug-linker stability of an anti-CD30 dipeptide-linked auristatin immunoconjugate. Clin. Cancer Res. 11, 843–852 (2005).
Baloglu, E. et al. Synthesis and biological evaluation of novel taxoids designed for targeted delivery to tumors. Bioorg. Med. Chem. Lett. 14, 5885–5888 (2004).
Mandler, R., Kobayashi, H., Hinson, E. R., Brechbiel, M. W. & Waldmann, T. A. Herceptin-geldanamycin immunoconjugates: pharmacokinetics, biodistribution, and enhanced antitumor activity. Cancer Res. 64, 1460–1467 (2004).
Frankel, A. E. New anti-T cell immunotoxins for the clinic. Leuk. Res. 29, 249–251 (2005).
Frankel, A. E., Kreitman, R. J. & Sausville, E. A. Targeted toxins. Clin. Cancer Res. 6, 326–334 (2000).
Thorpe, P. E. et al. Improved antitumor effects of immunotoxins prepared with deglycosylated ricin A-chain and hindered disulfide linkages. Cancer Res. 48, 6396–6403 (1988).
Hexham, J. M. et al. Influence of relative binding affinity on efficacy in a panel of anti-CD3 scFv immunotoxins. Mol. Immunol. 38, 397–408 (2001).
Posey, J. A. et al. A phase I trial of the single-chain immunotoxin SGN-10 (BR96 sFv-PE40) in patients with advanced solid tumors. Clin. Cancer Res. 8, 3092–3099 (2002).
Frankel, A. E. Reducing the immune response to immunotoxin. Clin. Cancer Res. 10, 13–15 (2004). This paper demonstrates the possibility of reducing the immunogenicity of immunotoxins.
Youn, Y. S., Na, D. H., Yoo, S. D., Song, S. C. & Lee, K. C. Carbohydrate-specifically polyethylene glycol-modified ricin A-chain with improved therapeutic potential. Int. J. Biochem. Cell Biol 37, 1525–1533 (2005).
Arndt, M. A., Krauss, J., Vu, B. K., Newton, D. L. & Rybak, S. M. A dimeric angiogenin immunofusion protein mediates selective toxicity toward CD22+ tumor cells. J. Immunother. 28, 245–251 (2005).
Krauss, J., Arndt, M. A., Vu, B. K., Newton, D. L. & Rybak, S. M. Targeting malignant B-cell lymphoma with a humanized anti-CD22 scFv-angiogenin immunoenzymedouble dagger. Br. J. Haematol. 128, 602–609 (2005).
De Lorenzo, C. et al. A fully human antitumor immunoRNase selective for ErbB-2-positive carcinomas. Cancer Res. 64, 4870–4874 (2004).
Liu, C. et al. Eradication of large colon tumor xenografts by targeted delivery of maytansinoids. Proc. Natl Acad. Sci. USA 93, 8618–8623 (1996).
Bagshawe, K. D. Antibody directed enzymes revive anti-cancer prodrugs concept. Br. J. Cancer 56, 531–532 (1987).
Denny, W. A. Tumor-activated prodrugs-a new approach to cancer therapy. Cancer Invest. 22, 604–619 (2004).
Chester, K. et al. Engineering antibodies for clinical applications in cancer. Tumour. Biol. 25, 91–98 (2004).
Senter, P. D. & Springer, C. J. Selective activation of anticancer prodrugs by monoclonal antibody-enzyme conjugates. Adv. Drug Deliv. Rev 53, 247–264 (2001). A detailed review on antibody dependent enzyme prodrug therapy.
Sharma, S. K., Bagshawe, K. D. & Begent, R. H. Advances in antibody-directed enzyme prodrug therapy. Curr. Opin. Investig. Drugs 6, 611–615 (2005).
Van Pel, A. & Boon, T. Protection against a nonimmunogenic mouse leukemia by an immunogenic variant obtained by mutagenesis. Proc. Natl Acad. Sci. USA 79, 4718–4722 (1982).
Rosenberg, S. A., Yang, J. C., White, D. E. & Steinberg, S. M. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann. Surg. 228, 307–319 (1998).
Robinson, B. W. et al. Cytokine gene therapy or infusion as treatment for solid human cancer. J Immunother. 21, 211–217 (1998).
Soiffer, R. J., Robertson, M. J., Murray, C., Cochran, K. & Ritz, J. Interleukin-12 augments cytolytic activity of peripheral blood lymphocytes from patients with hematologic and solid malignancies. Blood 82, 2790–2796 (1993).
Manusama, E. R. et al. Tumor necrosis factor-α in isolated perfusion systems in the treatment of cancer: the Rotterdam preclinical-clinical program. Semin. Surg. Oncol. 14, 232–237 (1998).
Keilholz, U. et al. Recombinant interleukin-2-based treatments for advanced melanoma: the experience of the European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. Cancer J. Sci. Am. 3 (Suppl. 1), S22–S28 (1997).
Cohen, J. IL-12 deaths: explanation and a puzzle. Science 270, 908 (1995).
Mattijssen, V. et al. Intratumoral PEG-interleukin-2 therapy in patients with locoregionally recurrent head and neck squamous-cell carcinoma. Ann. Oncol. 5, 957–960 (1994).
Lienard, D., Ewalenko, P., Delmotte, J. J., Renard, N. & Lejeune, F. J. High-dose recombinant tumor necrosis factor α in combination with interferon γ and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin. Oncol. 10, 52–60 (1992).
Hoogenboom, H. R., Volckaert, G. & Raus, J. C. Construction and expression of antibody-tumor necrosis factor fusion proteins. Mol. Immunol. 28, 1027–1037 (1991).
Gillies, S. D., Young, D., Lo, K. M., Foley, S. F. & Reisfeld, R. A. Expression of genetically engineered immunoconjugates of lymphotoxin and a chimeric anti-ganglioside GD2 antibody. Hybridoma 10, 347–356 (1991).
Gillies, S. D., Young, D., Lo, K. M. & Roberts, S. Biological activity and in vivo clearance of antitumor antibody/cytokine fusion proteins. Bioconjug. Chem. 4, 230–235 (1993). A description of the biological activity of several immunocytokines.
Pancook, J. D., Becker, J. C., Gillies, S. D. & Reisfeld, R. A. Eradication of established hepatic human neuroblastoma metastases in mice with severe combined immunodeficiency by antibody-targeted interleukin-2. Cancer Immunol. Immunother. 42, 88–92 (1996).
Becker, J. C., Pancook, J. D., Gillies, S. D., Mendelsohn, J. & Reisfeld, R. A. Eradication of human hepatic and pulmonary melanoma metastases in SCID mice by antibody-interleukin 2 fusion proteins. Proc. Natl Acad. Sci. USA 93, 2702–2707 (1996).
Xiang, R. et al. Elimination of established murine colon carcinoma metastases by antibody-interleukin 2 fusion protein therapy. Cancer Res. 57, 4948–4955 (1997).
Kendra, K. et al. Pharmacokinetics and stability of the ch14.18-interleukin-2 fusion protein in mice. Cancer Immunol. Immunother. 48, 219–229 (1999).
Becker, J. C., Pancook, J. D., Gillies, S. D., Furukawa, K. & Reisfeld, R. A. T cell-mediated eradication of murine metastatic melanoma induced by targeted interleukin 2 therapy. J. Exp. Med. 183, 2361–2366 (1996).
Lode, H. N. et al. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood 91, 1706–1715 (1998).
Niethammer, A. G. et al. Targeted interleukin 2 therapy enhances protective immunity induced by an autologous oral DNA vaccine against murine melanoma. Cancer Res. 61, 6178–6184 (2001).
Harvill, E. T., Fleming, J. M. & Morrison, S. L. In vivo properties of an IgG3-IL-2 fusion protein. A general strategy for immune potentiation. J. Immunol. 157, 3165–3170 (1996).
Dela Cruz, J. S. et al. Protein vaccination with the HER2/neu extracellular domain plus anti-HER2/neu antibody-cytokine fusion proteins induces a protective anti-HER2/neu immune response in mice. Vaccine 21, 1317–1326 (2003).
Dela Cruz, J. S., Morrison, S. L. & Penichet, M. L. Insights into the mechanism of anti-tumor immunity in mice vaccinated with the human HER2/neu extracellular domain plus anti-HER2/neu IgG3-(IL-2) or anti-HER2/neu IgG3-(GM-CSF) fusion protein. Vaccine (2005). This paper describes an approach to induce immune responses to soluble antigen by immunocytokines.
King, D. M. et al. Phase I clinical trial of the immunocytokine EMD 273063 in melanoma patients. J. Clin. Oncol. 22, 4463–4473 (2004). Results of the first phase I study of an IL-2 immunocytokine.
Ko, Y. J. et al. Safety, pharmacokinetics, and biological pharmacodynamics of the immunocytokine EMD 273066 (huKS-IL2): results of a phase I trial in patients with prostate cancer. J. Immunother. 27, 232–239 (2004).
Dela Cruz, J. S., Trinh, K. R., Morrison, S. L. & Penichet, M. L. Recombinant anti-human HER2/neu IgG3-(GM-CSF) fusion protein retains antigen specificity and cytokine function and demonstrates antitumor activity. J. Immunol. 165, 5112–5121 (2000).
Reisfeld, R. A., Gillies, S. D., Mendelsohn, J., Varki, N. M. & Becker, J. C. Involvement of B lymphocytes in the growth inhibition of human pulmonary melanoma metastases in athymic nu/nu mice by an antibody- lymphotoxin fusion protein. Cancer Res 56, 1707–1712 (1996).
Metelitsa, L. S. et al. Antidisialoganglioside/granulocyte macrophage-colony-stimulating factor fusion protein facilitates neutrophil antibody-dependent cellular cytotoxicity and depends on FcγRII (CD32) and Mac-1 (CD11b/CD18) for enhanced effector cell adhesion and azurophil granule exocytosis. Blood 99, 4166–4173 (2002).
Borsi, L. et al. Selective targeted delivery of TNFα to tumor blood vessels. Blood 102, 4384–4392 (2003).
Ebbinghaus, C. et al. Engineered vascular-targeting antibody-interferon-γ fusion protein for cancer therapy. Int. J. Cancer 116, 304–313 (2005).
Holliger, P. & Hudson, P. J. Engineered antibody fragments and the rise of single domains. Nature Biotechnol. 23, 1126–1136 (2005).
Schrama, D. et al. Targeting of lymphotoxin-α to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid-like tissue. Immunity. 14, 111–121 (2001).
Halin, C. et al. Synergistic therapeutic effects of a tumor targeting antibody fragment, fused to interleukin 12 and to tumor necrosis factor α. Cancer Res. 63, 3202–3210 (2003).
Hombach, A., Heuser, C. & Abken, H. Simultaneous targeting of IL2 and IL12 to Hodgkin's lymphoma cells enhances activation of resting NK cells and tumor cell lysis. Int. J. Cancer 115, 241–247 (2005).
Hofmeister, V., Vetter, C., Schrama, D., Bröcker, E. B. & Becker, J. C. Tumor stroma-associated antigens for anti-cancer immunotherapy. Cancer Immunol. Immunother. 1–14 (2005).
Shimizu, M., Yoshimoto, T., Nagata, S. & Matsuzawa, A. A trial to kill tumor cells through Fas (CD95)-mediated apoptosis in vivo. Biochem. Biophys. Res. Commun. 228, 375–379 (1996).
Timmer, T., de Vries, E. G. & de Jong, S. Fas receptor-mediated apoptosis: a clinical application? J. Pathol. 196, 125–134 (2002).
Schneider, P. et al. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J. Exp. Med. 187, 1205–1213 (1998).
Samel, D. et al. Generation of a FasL-based proapoptotic fusion protein devoid of systemic toxicity due to cell-surface antigen-restricted activation. J. Biol. Chem. 278, 32077–32082 (2003).
Bremer, E. et al. Target cell-restricted apoptosis induction of acute leukemic T cells by a recombinant tumor necrosis factor-related apoptosis-inducing ligand fusion protein with specificity for human CD7. Cancer Res. 65, 3380–3388 (2005).
Bremer, E. et al. Simultaneous inhibition of EGFR signaling and enhanced activation of TRAIL-R-mediated apoptosis induction by an scFv: sTRAIL fusion protein with specificity for human EGFR. J. Biol. Chem. (2005).
Ou, X. et al. Antitumor effects of radioiodinated antisense oligonuclide mediated by VIP receptor. Cancer Gene Ther. 12, 313–320 (2005).
Brignole, C. et al. Neuroblastoma targeting by c-myb-selective antisense oligonucleotides entrapped in anti-GD(2) immunoliposome: immune cell-mediated anti-tumor activities. Cancer Lett. 228, 181–186 (2005).
Schiffelers, R. M. et al. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 32, e149 (2004).
Song, E. et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nature Biotechnol. 23, 709–717 (2005). A study demonstrating the feasibility of antibody mediated, cell-type specific siRNA targeting.
Moro, M. et al. Induction of therapeutic T-cell immunity by tumor targeting with soluble recombinant B7-immunoglobulin costimulatory molecules. Cancer Res. 59, 2650–2656 (1999).
Grosse-Hovest, L. et al. A recombinant bispecific single-chain antibody induces targeted, supra-agonistic CD28-stimulation and tumor cell killing. Eur. J. Immunol. 33, 1334–1340 (2003).
Kipriyanov, S. M., Moldenhauer, G., Strauss, G. & Little, M. Bispecific CD3 x CD19 diabody for T cell-mediated lysis of malignant human B cells. Int. J. Cancer 77, 763–772 (1998).
Bruenke, J. et al. Effective lysis of lymphoma cells with a stabilised bispecific single-chain Fv antibody against CD19 and FcγRIII (CD16). Br. J. Haematol. 130, 218–228 (2005).
Kipriyanov, S. M. et al. Synergistic antitumor effect of bispecific CD19 x CD3 and CD19 x CD16 diabodies in a preclinical model of non-Hodgkin's lymphoma. J. Immunol 169, 137–144 (2002).
Gao, Y. et al. Efficient inhibition of multidrug-resistant human tumors with a recombinant bispecific anti-P-glycoprotein x anti-CD3 diabody. Leukemia 18, 513–520 (2004).
Wolf, E., Hofmeister, R., Kufer, P., Schlereth, B. & Baeuerle, P. A. BiTEs: bispecific antibody constructs with unique anti-tumor activity. Drug Discov. Today 10, 1237–1244 (2005). A review of particular bispecific antibody constructs mediating anti-tumor effects by activating T cells.
Wang, X. B. et al. A new recombinant single chain trispecific antibody recruits T lymphocytes to kill CEA (carcinoma embryonic antigen) positive tumor cells in vitro efficiently. J. Biochem. (Tokyo) 135, 555–565 (2004).
Harris, M. Monoclonal antibodies as therapeutic agents for cancer. Lancet Oncol. 5, 292–302 (2004).
Winter, G., Griffiths, A. D., Hawkins, R. E. & Hoogenboom, H. R. Making antibodies by phage display technology. Annu. Rev. Immunol 12, 433–455 (1994).
Stohrer, M., Boucher, Y., Stangassinger, M. & Jain, R. K. Oncotic pressure in solid tumors is elevated. Cancer Res. 60, 4251–4255 (2000).
Yokota, T., Milenic, D. E., Whitlow, M. & Schlom, J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 52, 3402–3408 (1992).
Adams, G. P. et al. Increased affinity leads to improved selective tumor delivery of single-chain Fv antibodies. Cancer Res. 58, 485–490 (1998).
Neri, D. & Bicknell, R. Tumour vascular targeting. Nature Rev. Cancer 5, 436–446 (2005). This review describes the anti-tumor effect of vascular targeting and strategies to identify putative tumor vascular targets.
Oh, P. et al. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature 429, 629–635 (2004).
Gillies, S. D., Reilly, E. B., Lo, K. M. & Reisfeld, R. A. Antibody-targeted interleukin 2 stimulates T-cell killing of autologous tumor cells. Proc. Natl Acad. Sci. USA 89, 1428–1432 (1992).
thor Straten, P. et al. Activation of preexisting T cell clones by targeted interleukin 2 therapy. Proc. Natl Acad. Sci. USA 95, 8785–8790 (1998).
Lode, H. N. et al. Melanoma immunotherapy by targeted IL-2 depends on CD4(+) T-cell help mediated by CD40/CD40L interaction. J. Clin. Invest. 105, 1623–1630 (2000).
Schrama, D. et al. Therapeutic efficacy of tumor-targeted IL2 in LTα (−/−) mice depends on conditioned T cells. Cancer Immunol. Immunother. 1–6 (2005).
Neal, Z. C. et al. Enhanced activity of hu14.18-IL2 immunocytokine against murine NXS2 neuroblastoma when combined with interleukin 2 therapy. Clin. Cancer Res. 10, 4839–4847 (2004).
Eggert, A. O. et al. Specific peptide-mediated immunity against established melanoma tumors with dendritic cells requires IL-2 and fetal calf serum-free cell culture. Eur. J. Immunol 32, 122–127 (2002).
Schrama, D. et al. Shift from systemic to site-specific memory by tumor-targeted IL-2. J. Immunol. 172, 5843–5850 (2004).
Penichet, M. L., Harvill, E. T. & Morrison, S. L. An IgG3–IL-2 fusion protein recognizing a murine B cell lymphoma exhibits effective tumor imaging and antitumor activity. J. Interferon Cytokine Res. 18, 597–607 (1998).
Wu, A. M. & Senter, P. D. Arming antibodies: prospects and challenges for immunoconjugates. Nature Biotechnol. 23, 1137–1146 (2005).
Francis, R. J. et al. A phase I trial of antibody directed enzyme prodrug therapy (ADEPT) in patients with advanced colorectal carcinoma or other CEA producing tumours. Br. J. Cancer 87, 600–607 (2002).
Acknowledgements
The authors thank E.B. Bröcker for her unfailing support of their scientific work. The authors work is supported by Deutsche Forschungagemeinschaft and Wilhelm-Sander-Stiftung grants to J.C.B., as well as by Deutsche Krebshilfe grant to D.S.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- P-glycoprotein transmembrane pump
-
P-glycoprotein is a transmembrane energy-dependent drug efflux pump encoded by the multidrug resistance gene-1 in humans. By reducing intracellular drug levels P-glycoprotein can contribute to multidrug resistance to chemotherapeutics.
- Antifolates
-
A substance that blocks the activity of folic acid, which is an important factor in the synthesis of nucleic acids and proteins.
- Vinca alkaloids
-
Indolalkaloids previously isolated from the evergreen Vinca rosea that are antimitotic and antimicrotubule agents.
- Anthracyclines
-
Antibiotic agents derived from cultures of the fungus Streptomyces peucetius. Their antitumour efficacy is mainly ascribed to the intercalation into DNA.
- Monomethyl auristatin E
-
A synthetically modified, highly cytotoxic derivate from the tubulin modifier auristatin E, which is intended to enhance efficacy and lower toxicity.
- Doxorubicin
-
An anthracycline isolated from Streptomyces peucetius and used for treatment of different kinds of cancer. It binds to DNA and inhibits reverse transcriptase and RNA polymerase.
- DM1 and DM4
-
Chemical derivates of maytansinoid which inhibit microtubule function and demonstrate high cytotoxic potency.
- CC-1065
-
Chemotherapeutic drug with high cytotoxic potency isolated from Streptomyces zelensis which binds very strongly to DNA and inhibits both thymidine kinase and DNA polymerase-α activities.
- Second-generation taxanes
-
The first taxanes were generated from the yew tree (Taxus spp.). Taxanes stabilize microtubules and thereby prevent mitosis. Second-generation taxanes were discovered by structure–activity relationship studies and are highly active against drug-resistant cancer cell lines.
- Geldanamycin
-
Geldanamycin is a benzoquinone ansamycin originally isolated from Streptomyces hygroscopicus. It possesses high cytotoxic potency and exerts its toxicity by binding to the chaperone heat-shock protein 90, which in turn destabilizes several key enzymes regulating essential cellular functions.
- Xenograft tumour model
-
Human tumours grown in immunocompromised mice.
- Vascular leak syndrome
-
Increased vascular permeability causes extravasation of fluids and proteins which results in interstitial oedema and organ failure.
- Angiogenin
-
An ubiquitously secreted enzyme that belongs to the pancreatic RNase A superfamily of proteins. When targeted into cells it can completely abolish intracellular protein synthesis by cleaving intracellular tRNA substrates.
- Lymphotoxin-α
-
An immunoregulatory cytokine that belongs to the tumour-necrosis factor ligand superfamily. This cytokine plays an important role in the initiation of secondary lymphoid tissue.
- Diabodies
-
A scFV fragment with a linker of 3–12 amino acids between VH and VL cannot fold into a functional Fv domain and instead associates with a second scFv molecule to form a bivalent dimer. The association of two different scFV fragments results in a bispecific diabody.
- Annexin A1
-
Annexin A1 is a member of the multigene family of Ca2+-regulated phospholipid-binding and membrane-binding proteins. Annexins are involved in cell proliferation, signal transduction, membrane aggregation and vesicle fusion during endocytic vesicular transport.
Rights and permissions
About this article
Cite this article
Schrama, D., Reisfeld, R. & Becker, J. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov 5, 147–159 (2006). https://doi.org/10.1038/nrd1957
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd1957
This article is cited by
-
A novel strategy to generate immunocytokines with activity-on-demand using small molecule inhibitors
EMBO Molecular Medicine (2024)
-
Delivery of Corn-Derived Nanoparticles with Anticancer Activity to Tumor Tissues by Modification with Polyethylene Glycol for Cancer Therapy
Pharmaceutical Research (2023)
-
The past decade of Genentech experience in elucidation of novel reaction mechanisms in drug metabolism
Medicinal Chemistry Research (2023)
-
Antibody–drug conjugate as targeted therapeutics against hepatocellular carcinoma: preclinical studies and clinical relevance
Clinical and Translational Oncology (2022)
-
Recombinant immunotoxins development for HER2-based targeted cancer therapies
Cancer Cell International (2021)