Abstract
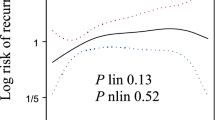
Since it was first described in 1981, CA125 has held an important role in monitoring patients with ovarian cancer. CA125 is elevated in 80% of patients with epithelial ovarian cancer at initial diagnosis and correlates well with response to therapy. CA125 monitoring is used for the follow up of patients with epithelial ovarian cancer, and elevations in CA125 measurements often antedate any signs, symptoms or radiographic evidence of disease by several months. Unfortunately, data favoring early therapeutic intervention for recurrent ovarian cancer is lacking, especially in patients with isolated CA125 elevations. In asymptomatic patients, elevations in CA125 have been associated with considerable anxiety and deterioration in quality of life without any significant gains in survival. Patients with ovarian cancer should, therefore, be counseled regarding the advantages and shortcomings of intensive CA125 testing. While some patients may benefit from early detection of recurrent disease and be candidates for secondary cytoreductive surgery, others may choose to delay therapy until they develop symptoms of disease recurrence. The results of a clinical trial suggest that withholding treatment in the event of isolated rising CA125 levels will not negatively impact these patients overall survival, highlighting the need for improved salvage therapies for recurrent ovarian cancer.
Key Points
-
Despite years of research the function of CA125 remains elusive
-
CA125 correlates well with response to initial therapy but suffers from poor specificity for the diagnosis of early ovarian cancer
-
In the follow-up of patients with epithelial ovarian cancer, elevations in CA125 often antedate any signs, symptoms or radiographic evidence of disease by several months
-
Data favoring early therapy for recurrent ovarian cancer are lacking especially in asymptomatic patients with isolated elevations in their CA125 measurements
-
Patients with epithelial ovarian cancer should be appropriately counseled regarding the advantages and shortcomings of CA125 testing
-
Patients may choose to delay therapy until they develop signs and symptoms of disease recurrence in the event of isolated rising CA125 levels
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Bast, R. C. Jr et al. Reactivity of a monoclonal antibody with human ovarian carcinoma. J. Clin. Invest. 6 8, 1331–1337 (1981).
Bast, R. C. Jr et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N. Engl. J. Med. 309, 883–887 (1983).
Partridge, E. et al. Results from four rounds of ovarian cancer screening in a randomized trial. Obstet. Gynecol. 113, 775–782 (2009).
Menon, U. et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol. 10, 327–340 (2009).
Rustin, G. J., van der Burg, M. E., on behalf of MRC and EORTC collaborators. A randomized trial in ovarian cancer (OC) of early treatment of relapse based on CA125 level alone versus delayed treatment based on conventional clinical indicators (MRC OV05/EORTC 55955 trials) [abstract]. J. Clin. Oncol. 27 (Suppl. 18s), a1 (2009).
Menczer, J. Re: “clinical implications of a rising serum CA-125 within the normal range in patients with epithelial ovarian cancer: a preliminary investigation”. Gynecol. Oncol. 96, 906–907; author reply 907 (2005).
Armstrong, D. K. Relapsed ovarian cancer: challenges and management strategies for a chronic disease. Oncologist 7 (Suppl. 5), 20–28 (2002).
O'Brien, T. J. et al. The CA 125 gene: an extracellular superstructure dominated by repeat sequences. Tumour Biol. 22, 348–366 (2001).
Yin, B. W. & Lloyd, K. O. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J. Biol. Chem. 276, 27371–27375 (2001).
Bast, R. C. Jr et al. CA 125: the past and the future. Int. J. Biol. Markers 13, 179–187 (1998).
Kenemans, P., Verstraeten, A. A., van Kamp, G. J. & von Mensdorff-Pouilly, S. The second generation CA 125 assays. Ann. Med. 27, 107–113 (1995).
Kenemans, P., van Kamp, G. J., Oehr, P. & Verstraeten, R. A. Heterologous double-determinant immunoradiometric assay CA 125 II: reliable second-generation immunoassay for determining CA 125 in serum. Clin. Chem. 39, 2509–2513 (1993).
Gubbels, J. A. et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol. Cancer 5, 50 (2006).
Rump, A. et al. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J. Biol. Chem. 279, 9190–9198 (2004).
Kaneko, O. et al. A binding domain on mesothelin for CA125/MUC16. J. Biol. Chem. 284, 3739–3749 (2009).
Thapi, D. et al. MUC16 gene expression in human ovarian cancer cells and cisplatin resistance. AACR Meeting Abstracts, a881 (2006).
Thapi, D. et al. MUC16 gene up-regulates gene transcripts associated with cisplatin resistance in human ovarian cancer cells. AACR Meeting Abstracts, a2327 (2007).
Hawkins, R. E., Roberts, K., Wiltshaw, E., Mundy, J. & McCready, V. R. The clinical correlates of serum CA125 in 169 patients with epithelial ovarian carcinoma. Br. J. Cancer 60, 634–637 (1989).
[No authors listed] Chemotherapy for advanced ovarian cancer. Advanced Ovarian Cancer Trialists Group. Cochrane Database of Systematic Reviews, Issue 1. Art No: CD001418. doi: 10.1002/14651858.CD001418 (2000).
Krivak, T. C., Tian, C., Rose, G. S., Armstrong, D. K. & Maxwell, G. L. A Gynecologic Oncology Group Study of serum CA-125 levels in patients with stage III optimally debulked ovarian cancer treated with intraperitoneal compared to intravenous chemotherapy: an analysis of patients enrolled in GOG 172. Gynecol. Oncol. 115, 81–85 (2009).
Juretzka, M. M. et al. CA125 level as a predictor of progression-free survival and overall survival in ovarian cancer patients with surgically defined disease status prior to the initiation of intraperitoneal consolidation therapy. Gynecol. Oncol. 104, 176–180 (2007).
Cannistra, S. A. Cancer of the ovary. N. Engl. J. Med. 351, 2519–2529 (2004).
International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet 360, 505–515 (2002).
Ozols, R. F. et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J. Clin. Oncol. 21, 3194–3200 (2003).
Rustin, G. J., Marples, M., Nelstrop, A. E., Mahmoudi, M. & Meyer, T. Use of CA-125 to define progression of ovarian cancer in patients with persistently elevated levels. J. Clin. Oncol. 19, 4054–4057 (2001).
Vergote, I. et al. Re: new guidelines to evaluate the response to treatment in solid tumors [ovarian cancer]. Gynecologic Cancer Intergroup. J. Natl Cancer Inst. 92, 1534–1535 (2000).
Tuxen, M. K., Soletormos, G., Rustin, G. J., Nelstrop, A. E. & Dombernowsky, P. Biological variation and analytical imprecision of CA 125 in patients with ovarian cancer. Scand. J. Clin. Lab Invest. 60, 713–721 (2000).
Bridgewater, J. A. et al. Comparison of standard and CA-125 response criteria in patients with epithelial ovarian cancer treated with platinum or paclitaxel. J. Clin. Oncol. 17, 501–508 (1999).
Rustin, G. J., Nelstrop, A. E., Tuxen, M. K. & Lambert, H. E. Defining progression of ovarian carcinoma during follow-up according to CA 125: a North Thames Ovary Group Study. Ann. Oncol. 7, 361–364 (1996).
Tuxen, M. K., Soletormos, G. & Dombernowsky, P. Serum tumor marker CA 125 for monitoring ovarian cancer during follow-up. Scand. J. Clin. Lab Invest. 62, 177–188 (2002).
Therasse, P. et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl Cancer Inst. 92, 205–216 (2000).
Wilder, J. L. et al. Clinical implications of a rising serum CA-125 within the normal range in patients with epithelial ovarian cancer: a preliminary investigation. Gynecol. Oncol. 89, 233–235 (2003).
Santillan, A. et al. Risk of epithelial ovarian cancer recurrence in patients with rising serum CA-125 levels within the normal range. J. Clin. Oncol. 23, 9338–9343 (2005).
Gadducci, A., Cosio, S., Conte, P. F. & Genazzani, A. R. Consolidation and maintenance treatments for patients with advanced epithelial ovarian cancer in complete response after first-line chemotherapy: a review of the literature. Crit. Rev. Oncol. Hematol. 55, 153–166 (2005).
Eisenhauer, E. A., Vermorken, J. B. & van Glabbeke, M. Predictors of response to subsequent chemotherapy in platinum pretreated ovarian cancer: a multivariate analysis of 704 patients. Ann. Oncol. 8, 963–968 (1997).
Fleming, L. W. Playing the waiting game ... the asymptomatic patient with recurrent ovarian cancer detected only by rising Ca125 levels. Scott. Med. J. 46, 81–83 (2001).
Hacker, N. F. & Friedlander, M. Treatment of recurrent ovarian cancer. Chang. Gung. Med. J. 27, 570–577 (2004).
Rubin, S. C., Randall, T. C., Armstrong, K. A., Chi, D. S. & Hoskins, W. J. Ten-year follow-up of ovarian cancer patients after second-look laparotomy with negative findings. Obstet. Gynecol. 93, 21–24 (1999).
Rubin, S. C. et al. Prognostic factors for recurrence following negative second-look laparotomy in ovarian cancer patients treated with platinum-based chemotherapy. Gynecol. Oncol. 42, 137–141 (1991).
Obermair, A. & Sevelda, P. Impact of second look laparotomy and secondary cytoreductive surgery at second-look laparotomy in ovarian cancer patients. Acta Obstet. Gynecol. Scand. 80, 432–436 (2001).
Greer, B. E. et al. Implications of second-look laparotomy in the context of optimally resected stage III ovarian cancer: a non-randomized comparison using an explanatory analysis: a Gynecologic Oncology Group study. Gynecol. Oncol. 99, 71–79 (2005).
Hurteau, J. et al. A randomized phase III trial of tamoxifen versus thalidomide in women with biochemical-recurrent-only epithelial ovarian, fallopian tube, or primary peritoneal carcinoma with an evaluation of serum vascular endothelial growth factor: A Gynecologic Oncology Group study. Gynecol. Oncol. 116 (Suppl.) a2 (2010).
Janicke, F. et al. Radical surgical procedure improves survival time in patients with recurrent ovarian cancer. Cancer 70, 2129–2136 (1992).
Segna, R. A., Dottino, P. R., Mandeli, J. P., Konsker, K. & Cohen, C. J. Secondary cytoreduction for ovarian cancer following cisplatin therapy. J. Clin. Oncol. 11, 434–439 (1993).
Gadducci, A. et al. Complete salvage surgical cytoreduction improves further survival of patients with late recurrent ovarian cancer. Gynecol. Oncol. 79, 344–349 (2000).
Salani, R. et al. Secondary cytoreductive surgery for localized, recurrent epithelial ovarian cancer: analysis of prognostic factors and survival outcome. Cancer 109, 685–691 (2007).
Tay, E. H., Grant, P. T., Gebski, V. & Hacker, N. F. Secondary cytoreductive surgery for recurrent epithelial ovarian cancer. Obstet. Gynecol. 99, 1008–1013 (2002).
Ayhan, A. et al. The role of secondary cytoreduction in the treatment of ovarian cancer: Hacettepe University experience. Am. J. Obstet. Gynecol. 194, 49–56 (2006).
Chi, D. S. et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer 106, 1933–1939 (2006).
Onda, T. et al. Secondary cytoreductive surgery for recurrent epithelial ovarian carcinoma: proposal for patients selection. Br. J. Cancer 92, 1026–1032 (2005).
Tebes, S. J. et al. Cytoreductive surgery for patients with recurrent epithelial ovarian carcinoma. Gynecol. Oncol. 106, 482–487 (2007).
Scarabelli, C., Gallo, A. & Carbone, A. Secondary cytoreductive surgery for patients with recurrent epithelial ovarian carcinoma. Gynecol. Oncol. 83, 504–512 (2001).
Eisenkop, S. M., Friedman, R. L. & Spirtos, N. M. The role of secondary cytoreductive surgery in the treatment of patients with recurrent epithelial ovarian carcinoma. Cancer 88, 144–153 (2000).
Harter, P. et al. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann. Surg. Oncol. 13, 1702–1710 (2006).
Zang, R. Y. et al. Secondary cytoreductive surgery for patients with relapsed epithelial ovarian carcinoma: who benefits? Cancer 100, 1152–1161 (2004).
Benedetti Panici, P. et al. Secondary cytoreductive surgery in patients with platinum-sensitive recurrent ovarian cancer. Ann. Surg. Oncol. 14, 1136–1142 (2007).
Karam, A. K. et al. Tertiary cytoreductive surgery in recurrent ovarian cancer: selection criteria and survival outcome. Gynecol. Oncol. 104, 377–380 (2007).
Guppy, A. E. & Rustin, G. J. CA125 response: can it replace the traditional response criteria in ovarian cancer? Oncologist 7, 437–443 (2002).
Parker, P. A. et al. The associations between knowledge, CA125 preoccupation, and distress in women with epithelial ovarian cancer. Gynecol. Oncol. 100, 495–500 (2006).
Havrilesky, L. J. et al. Evaluation of biomarker panels for early stage ovarian cancer detection and monitoring for disease recurrence. Gynecol. Oncol. 110, 374–382 (2008).
Allard, J., Somers, E., Theil, R. & Moore, R. G. Use of a novel biomarker HE4 for monitoring patients with epithelial ovarian cancer [abstract]. J. Clin. Oncol. 26, a5535 (2008).
[No authors listed] BiPar Sciences presents interim phase 2 results for PARP inhibitor BSI-201 at San Antonio Breast Cancer Symposium. Cancer Biol. Ther. 8, 2–3 (2009).
Burger, R. A., Sill, M. W., Monk, B. J., Greer, B. E. & Sorosky, J. I. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J. Clin. Oncol. 25, 5165–5171 (2007).
Rauh-Hain, J. A. & Penson, R. T. Potential benefit of sunitinib in recurrent and refractory ovarian clear cell adenocarcinoma. Int. J. Gynecol. Cancer 18, 934–936 (2008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Karam, A., Karlan, B. Ovarian cancer: the duplicity of CA125 measurement. Nat Rev Clin Oncol 7, 335–339 (2010). https://doi.org/10.1038/nrclinonc.2010.44
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2010.44
This article is cited by
-
Noninvasive serum N-glycans associated with ovarian cancer diagnosis and precancerous lesion prediction
Journal of Ovarian Research (2024)
-
Four-protein model for predicting prognostic risk of lung cancer
Frontiers of Medicine (2022)
-
Core fucosylated glycan-dependent inhibitory effect of QSOX1-S on invasion and metastasis of hepatocellular carcinoma
Cell Death Discovery (2019)
-
HE4 level in ascites may assess the ovarian cancer chemotherapeutic effect
Journal of Ovarian Research (2018)
-
Dynamic N-glycoproteome analysis of maize seedling leaves during de-etiolation using Concanavalin A lectin affinity chromatography and a nano-LC–MS/MS-based iTRAQ approach
Plant Cell Reports (2017)