Abstract
Systemic therapy has become an increasingly important component of treatment of advanced prostate cancer. In the past decade, important innovations have been achieved in the development of novel systemic hormonal therapies for the salvage treatment of metastatic castrate-resistant disease. These improvements have been accompanied by the broadening of potential indications for chemotherapy in castrate-resistant metastatic disease and the use of chemotherapy as an adjunct to the treatment of locally extensive tumors. These changes have begun to lead to improved outcomes, but at the expense of novel patterns of late toxic effects. We review the key steps in the recent evolution of systemic therapy of prostate cancer.
Key Points
-
Docetaxel-based chemotherapy provides a survival benefit for patients with metastatic disease; docetaxel increased median survival from 17.5 months to 19.2 months compared with mitoxantrone
-
Survival for metastatic disease has not improved much in the past decade; however, systemic hormone suppression in association with definitive local therapy has led to improved survival in node-positive patients
-
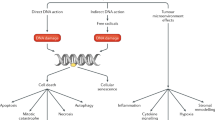
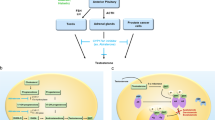
Although systemic androgen deprivation therapy causes biochemical and clinical castration and suppresses tumor growth, tumors may continue to be stimulated by androgens produced by the adrenal gland or the tumor
-
Abiraterone acetate is a novel hormonal therapy that binds CYP17A1 and irreversibly inhibits this enzyme, which prevents androgen formation; this agent is more specific than other secondary hormonal therapies
-
Abiraterone acetate is currently being evaluated in phase I–II trials; clinical and prostate-specific antigen responses have been reported
-
Many other agents have been tested in castration-resistant prostate cancer, including atrasentan, bevacizumab, sorafenib, erlotinib, gefitinib, calcitriol and doxercalciferol, but their role and utility have not yet been defined
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Jemal, A. et al. Cancer statistics, 2008. CA Cancer J. Clin. 58, 71–96 (2008).
Huggins, C. & Hodges, C. V. Studies on prostatic cancer I: the effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1, 293–297 (1941).
Berthold, D. R. et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J. Clin. Oncol. 26, 242–245 (2008).
Petrylak, D. P. et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 351, 1513–1520 (2004).
Sartor, A. O. et al. Antiandrogen withdrawal in castrate-refractory prostate cancer: a Southwest Oncology Group trial (SWOG 9426). Cancer 112, 2393–2400 (2008).
Small, E. J. et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J. Clin. Oncol. 22, 1025–1033 (2004).
Tannock, I. F. et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 351, 1502–1512 (2004).
Trachtenberg, J. & Pont, A. Ketoconazole therapy for advanced prostate cancer. Lancet 2, 433–435 (1984).
Drake, C. G. Immunotherapy for metastatic prostate cancer. Urol. Oncol. 26, 438–444 (2008).
Kiessling, A. et al. Advances in specific immunotherapy for prostate cancer. Eur. Urol. 53, 694–708 (2008).
Loose, D. S., Kan, P. B., Hirst, M. A., Marcus, R. A. & Feldman, D. Ketoconazole blocks adrenal steroidogenesis by inhibiting cytochrome P450-dependent enzymes. J. Clin. Invest. 71, 1495–1499 (1983).
Ryan, C. J. & Small, E. J. Role of secondary hormonal therapy in the management of recurrent prostate cancer. Urology 62 (Suppl. 1), 87–94 (2003).
Montgomery, R. B. et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 68, 4447–4454 (2008).
Mostaghel, E. A. et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 67, 5033–5041 (2007).
Stigliano, A. et al. Increased metastatic lymph node 64 and CYP17 expression are associated with high stage prostate cancer. J. Endocrinol. 194, 55–61 (2007).
Mohler, J. L. et al. The androgen axis in recurrent prostate cancer. Clin. Cancer Res. 10, 440–448 (2004).
Thomas, L. N. et al. Differential alterations in 5α-reductase type 1 and type 2 levels during development and progression of prostate cancer. Prostate 63, 231–239 (2005).
Hakki, T. & Bernhardt, R. CYP17- and CYP11B-dependent steroid hydroxylases as drug development targets. Pharmacol. Ther. 111, 27–52 (2006).
O'Donnell, A. et al. Hormonal impact of the 17α-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br. J. Cancer 90, 2317–2325 (2004).
Attard, G. et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J. Clin. Oncol. 26, 4563–4571 (2008).
Ryan, C. et al. Impact of prior ketoconazole therapy on response proportion to abiraterone acetate, a 17-α hydroxylase C17,20-lyase inhibitor in castration resistant prostate cancer (CRPC) [abstract]. J. Clin. Oncol. 26 (Suppl.), a5018 (2008).
Danila, D. C. et al. Abiraterone acetate and prednisone in patients (Pts) with progressive metastatic castration resistant prostate cancer (CRPC) after failure of docetaxel-based chemotherapy [abstract]. J. Clin. Oncol. 26 (Suppl.), a5019 (2008).
De Bono, J. S. et al. Anti-tumor activity of abiraterone acetate (AA), a CYP17 inhibitor of androgen synthesis, in chemotherapy naive and docetaxel pre-treated castration resistant prostate cancer (CRPC) [abstract]. J. Clin. Oncol. 26 (Suppl.), a5005 (2008).
Sternberg, C. N. et al. Phase III trial of satraplatin, an oral platinum plus prednisone vs. prednisone alone in patients with hormone-refractory prostate cancer. Oncology 68, 2–9 (2005).
Sartor, A. O. et al. Satraplatin in patients with advanced hormone-refractory prostate cancer (HRPC): overall survival (OS) results from the phase III satraplatin and prednisone against refractory cancer (SPARC) trial [abstract]. J. Clin. Oncol. 26 (Suppl.), a5003 (2008).
Armstrong, A. J. et al. A phase I–II study of docetaxel and atrasentan in men with castration-resistant metastatic prostate cancer. Clin. Cancer Res. 14, 6270–6276 (2008).
Attia, S. et al. Randomized, double-blinded phase II evaluation of docetaxel with or without doxercalciferol in patients with metastatic, androgen-independent prostate cancer. Clin. Cancer Res. 14, 2437–2443 (2008).
Boccardo, F. et al. Prednisone plus gefitinib versus prednisone plus placebo in the treatment of hormone-refractory prostate cancer: a randomized phase II trial. Oncology 74, 223–228 (2008).
Chan, J. S. et al. A phase II study of high-dose calcitriol combined with mitoxantrone and prednisone for androgen-independent prostate cancer. BJU Int. 102, 1601–1606 (2008).
Di Lorenzo, G. et al. Combination of bevacizumab and docetaxel in docetaxel-pretreated hormone-refractory prostate cancer: a phase 2 study. Eur. Urol. 54, 1089–1094 (2008).
Gravis, G. et al. Results from a monocentric phase II trial of erlotinib in patients with metastatic prostate cancer. Ann. Oncol. 19, 1624–1628 (2008).
Nelson, J. B. et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer 113, 2478–2487 (2008).
Chi, K. N. et al. A phase II study of sorafenib in patients with chemo-naive castration-resistant prostate cancer. Ann. Oncol. 19, 746–751 (2008).
Galsky, M. D. et al. Multi-institutional randomized phase II trial of the epothilone B analog ixabepilone (BMS-247550) with or without estramustine phosphate in patients with progressive castrate metastatic prostate cancer. J. Clin. Oncol. 23, 1439–1446 (2005).
Hahn, N. M. et al. A multicenter phase II study of pemetrexed as second-line chemotherapy for the treatment of hormone refractory prostate cancer (HRPC); Hoosier Oncology Group GU-0367 [abstract]. J. Clin. Oncol. 26 (Suppl.), a16019 (2008).
Hussain, A. et al. A phase IIa trial of weekly EPO906 in patients with hormone-refractory prostate cancer (HRPC) [abstract]. J. Clin. Oncol. 22 (Suppl.), a4563 (2004).
Lin, A. M. et al. Clinical outcome of taxane-resistant (TR) hormone refractory prostate cancer (HRPC) paients (pts) treated with subsequent chemotherapy (ixabepilone (Ix) or mitoxantrone/prednisone (MP) [abstract]. J. Clin. Oncol. 24 (Suppl.), a4558 (2006).
Saad, F. et al. Cancer treatment-induced bone loss in breast and prostate cancer. J. Clin. Oncol. 26, 5465–5476 (2008).
Bolla, M. et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 360, 103–106 (2002).
Bolla, M. et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N. Engl. J. Med. 337, 295–300 (1997).
Pilepich, M. V. et al. Phase III radiation therapy oncology group (RTOG) trial 86–10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int. J. Radiat. Oncol. Biol. Phys. 50, 1243–1252 (2001).
Roach, M. 3rd et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J. Clin. Oncol. 26, 585–591 (2008).
Kelly, W. K. et al. Multicenter phase 2 study of neoadjuvant paclitaxel, estramustine phosphate, and carboplatin plus androgen deprivation before radiation therapy in patients with unfavorable-risk localized prostate cancer: results of Cancer and Leukemia Group B 99811. Cancer 113, 3137–3145 (2008).
Klotz, L. H. et al. Long-term follow-up of a randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. J. Urol. 170, 791–794 (2003).
Soloway, M. S. et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxM0 prostate cancer: 5-year results. J. Urol. 167, 112–116 (2002).
Kang, T. Y. et al. Functional heterogeneity of prostatic intraepithelial neoplasia: the duration of hormonal therapy influences the response. BJU Int. 99, 1024–1027 (2007).
Dreicer, R. et al. Phase II trial of neoadjuvant docetaxel before radical prostatectomy for locally advanced prostate cancer. Urology 63, 1138–1142 (2004).
Febbo, P. G. et al. Neoadjuvant docetaxel before radical prostatectomy in patients with high-risk localized prostate cancer. Clin. Cancer Res. 11, 5233–5240 (2005).
Friedman, J. et al. Neoadjuvant docetaxel and capecitabine in patients with high risk prostate cancer. J. Urol. 179, 911–916 (2008).
Garcia, J. A. et al. Clinical and biological effects of neoadjuvant sargramostim and thalidomide in patients with locally advanced prostate carcinoma. Clin. Cancer Res. 14, 3052–3059 (2008).
Shepard, D. R. et al. Phase II trial of neoadjuvant nab-paclitaxel in high-risk patients with prostate cancer undergoing radical prostatectomy. J. Urol. 181, 1672–1677 (2009).
Hussain, M. et al. Neoadjuvant docetaxel and estramustine chemotherapy in high-risk/locally advanced prostate cancer. Urology 61, 774–780 (2003).
Chi, K. N. et al. Multicenter phase II study of combined neoadjuvant docetaxel and hormone therapy before radical prostatectomy for patients with high risk localized prostate cancer. J. Urol. 180, 565–570 (2008).
Prayer-Galetti, T. et al. Long-term follow-up of a neoadjuvant chemohormonal taxane-based phase II trial before radical prostatectomy in patients with non-metastatic high-risk prostate cancer. BJU Int. 100, 274–280 (2007).
Sella, A. et al. Neoadjuvant chemohormonal therapy in poor-prognosis localized prostate cancer. Urology 71, 323–327 (2008).
Eastham, J. A., Kelly, W. K., Grossfeld, G. D. & Small, E. J. Cancer and Leukemia Group B (CALGB) 90203: a randomized phase 3 study of radical prostatectomy alone versus estramustine and docetaxel before radical prostatectomy for patients with high-risk localized disease. Urology 62 (Suppl. 1), 55–62 (2003).
Pilepich, M. V. et al. Phase III trial of androgen suppression using goserelin in unfavorable-prognosis carcinoma of the prostate treated with definitive radiotherapy: report of Radiation Therapy Oncology Group Protocol 85–31. J. Clin. Oncol. 15, 1013–1021 (1997).
Messing, E. M. et al. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N. Engl. J. Med. 341, 1781–1788 (1999).
Messing, E. M. et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 7, 472–479 (2006).
Hanks, G. E. et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92–02. J. Clin. Oncol. 21, 3972–3978 (2003).
Kibel, A. S. et al. Adjuvant weekly docetaxel for patients with high risk prostate cancer after radical prostatectomy: a multi-institutional pilot study. J. Urol. 177, 1777–1781 (2007).
Flaig, T. W. et al. Randomization reveals unexpected acute leukemias in Southwest Oncology Group prostate cancer trial. J. Clin. Oncol. 26, 1532–1536 (2008).
Rosenthal, S. A. et al. Phase III multi-institutional trial of adjuvant chemotherapy with paclitaxel, estramustine, and oral etoposide combined with long-term androgen suppression therapy and radiotherapy versus long-term androgen suppression plus radiotherapy alone for high-risk prostate cancer: preliminary toxicity analysis of RTOG 99–02. Int. J. Radiat. Oncol. Biol. Phys. 73, 672–678 (2009).
Le Deley, M. C. et al. Anthracyclines, mitoxantrone, radiotherapy, and granulocyte colony-stimulating factor: risk factors for leukemia and myelodysplastic syndrome after breast cancer. J. Clin. Oncol. 25, 292–300 (2007).
Lu-Yao, G., Stukel, T. A. & Yao, S. L. Changing patterns in competing causes of death in men with prostate cancer: a population based study. J. Urol. 171, 2285–2290 (2004).
Basaria, S. Androgen deprivation therapy, insulin resistance and cardiovascular mortality: an inconvenient truth. J. Androl. 29, 534–539 (2008).
Basaria, S. et al. Long-term effects of androgen deprivation therapy in prostate cancer patients. Clin. Endocrinol. (Oxf.) 56, 779–786 (2002).
Basaria, S., Muller, D. C., Carducci, M. A., Egan, J. & Dobs, A. S. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer 106, 581–588 (2006).
Braga-Basaria, M. et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J. Clin. Oncol. 24, 3979–3983 (2006).
Bylow, K. et al. Falls and physical performance deficits in older patients with prostate cancer undergoing androgen deprivation therapy. Urology 72, 422–427 (2008).
Derweesh, I. H. et al. Risk of new-onset diabetes mellitus and worsening glycemic variables for established diabetes in men undergoing androgen-deprivation therapy for prostate cancer. BJU Int. 100, 1060–1065 (2007).
Efstathiou, J. A. et al. Cardiovascular mortality and duration of androgen deprivation for locally advanced prostate cancer: analysis of RTOG 92–02. Eur. Urol. 54, 816–823 (2008).
Efstathiou, J. A. et al. Cardiovascular mortality after androgen deprivation therapy for locally advanced prostate cancer: RTOG 85–31. J. Clin. Oncol. 27, 92–99 (2009).
Keating, N. L., O'Malley, A. J. & Smith, M. R. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J. Clin. Oncol. 24, 4448–4456 (2006).
Kiratli, B. J., Srinivas, S., Perkash, I. & Terris, M. K. Progressive decrease in bone density over 10 years of androgen deprivation therapy in patients with prostate cancer. Urology 57, 127–132 (2001).
Lage, M. J., Barber, B. L. & Markus, R. A. Association between androgen-deprivation therapy and incidence of diabetes among males with prostate cancer. Urology 70, 1104–1108 (2007).
Smith, M. R. et al. Diabetes and mortality in men with locally advanced prostate cancer: RTOG 92–02. J. Clin. Oncol. 26, 4333–4339 (2008).
Smith, M. R., Lee, H., Fallon, M. A. & Nathan, D. M. Adipocytokines, obesity, and insulin resistance during combined androgen blockade for prostate cancer. Urology 71, 318–322 (2008).
Smith, M. R., Lee, H. & Nathan, D. M. Insulin sensitivity during combined androgen blockade for prostate cancer. J. Clin. Endocrinol. Metab. 91, 1305–1308 (2006).
Yannucci, J., Manola, J., Garnick, M. B., Bhat, G. & Bubley, G. J. The effect of androgen deprivation therapy on fasting serum lipid and glucose parameters. J. Urol. 176, 520–525 (2006).
Satariano, W. A., Ragland, K. E. & van den Eeden, S. K. Cause of death in men diagnosed with prostate carcinoma. Cancer 83, 1180–1188 (1998).
Saigal, C. S. et al. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer 110, 1493–1500 (2007).
D'Amico, A. V. et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J. Clin. Oncol. 25, 2420–2425 (2007).
Tsai, H. K., D'Amico, A. V., Sadetsky, N., Chen, M. H. & Carroll, P. R. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J. Natl Cancer Inst. 99, 1516–1524 (2007).
Cooper, C. S., Campbell, C. & Jhavar, S. Mechanisms of disease: biomarkers and molecular targets from microarray gene expression studies in prostate cancer. Nat. Clin. Pract. Urol. 4, 677–687 (2007).
de Bono, J. S. et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 14, 6302–6309 (2008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Shepard, D., Raghavan, D. Innovations in the systemic therapy of prostate cancer. Nat Rev Clin Oncol 7, 13–21 (2010). https://doi.org/10.1038/nrclinonc.2009.187
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2009.187
This article is cited by
-
Altered CXCR3 isoform expression regulates prostate cancer cell migration and invasion
Molecular Cancer (2012)
-
BAG3 is required for IKKα nuclear translocation and emergence of castration resistant prostate cancer
Cell Death & Disease (2011)
-
[11C]Choline as pharmacodynamic marker for therapy response assessment in a prostate cancer xenograft model
European Journal of Nuclear Medicine and Molecular Imaging (2010)