Key Points
-
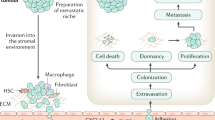
Common tumours, such as those of the breast, lung and prostate, frequently metastasize to bone, and in many patients with advanced disease the skeleton is the site of the most significant tumour burden.
-
There are different patterns of bone effects in patients with cancer, ranging from purely or mostly destructive or osteolytic (breast cancer, myeloma), to mostly bone-forming or osteoblastic (prostate cancer).
-
In the case of breast-cancer-causing osteolysis, the main mediator is parathyroid-hormone-related peptide (PTHrP), whereas, in osteoblastic lesions, known mediators include endothelin-1 and platelet-derived growth factor.
-
In osteolytic metastasis, there is a 'vicious cycle' in the bone microenvironment, whereby bi-directional interactions between tumour cells and osteoclasts lead to both osteolysis and tumour growth.
-
The molecular mechanisms that are responsible for this vicious cycle are now being clarified and involve tumour-cell production of PTHrP and bone-derived growth factors that are released as a consequence of increased bone resorption.
-
Bisphosphonates interrupt the vicious cycle and cause not only a reduction in osteolytic bone lesions, but also decrease the tumour burden in bone.
-
More-effective treatments for interruption of the vicious cycle are now being developed, including specifically neutralizing antibodies to PTHrP and more efficacious osteoclast inhibitors.
Abstract
The most common human cancers — lung, breast and prostate — have a great avidity for bone, leading to painful and untreatable consequences. What makes some cancers, but not others, metastasize to bone, and how do they alter its physiology? Some of the molecular mechanisms that are responsible have recently been identified, and provide new molecular targets for drug development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wingo, P. A., Tong, T. & Bolden, S. Cancer statistics, 1995. CA Cancer J. Clin. 45, 8–30 (1995).
Mantyh, P. W., Clohisy, D. R., Kotzenburg, M. & Hunt, S. P. Molecular mechanisms of cancer pain. Nature Rev. Cancer 2, 201–209 (2000).
Honore, P. et al. Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nature Med. 6, 521–528 (2000).Evidence that the bone-resorption inhibitor osteoprotegerin inhibits not just osteolysis but also bone pain in metastatic bone disease.
Boyde, A., Maconnachie, E., Reid, S. A., Delling, G. & Mundy, G. R. Scanning electron microscopy in bone pathology: review of methods, potential and applications. Scan. Electron Microsc., 1537–1554 (1986).This paper shows, by scanning electron microscopy, evidence of osteoclastic resorption pits at bone metastatic sites, indicating the importance of osteoclasts as cellular mediators of osteolysis.
Stewart, A. F. et al. Quantitative bone histomorphometry in humoral hypercalcemia of malignancy: uncoupling of bone cell activity. J. Clin. Endocrinol. Metab. 55, 219–227 (1982).Histomorphometric evidence of the abnormalities in osteoblastic and osteolytic activity that occurs in patients with bone metastases. This was shown by observing the impaired osteoblastic response to increased osteoclastic resorption.
Charhon, S. A. et al. Histomorphometric analysis of sclerotic bone metastases from prostatic carcinoma special reference to osteomalacia. Cancer 51, 918–924 (1983).
Garnero, P. et al. Markers of bone turnover for the management of patients with bone metastases from prostate cancer. Br. J. Cancer 82, 858–864 (2000).
Koga, H. et al. Use of bone turnover marker, pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP), in the assessment and monitoring of bone metastasis in prostate cancer. Prostate 39, 1–7 (1999).
Koizumi, M. et al. Dissociation of bone formation markers in bone metastasis of prostate cancer. Br. J. Cancer 75, 1601–1604 (1997).
Percival, R. C. et al. Biochemical and histological evidence that carcinoma of the prostate is associated with increased bone resorption. Eur. J. Surg. Oncol. 13, 41–49 (1987).One of the first studies to indicate that osteoblastic metastases are accompanied by increased bone resorption in patients with prostate cancer.
Yoshida, K. et al. Serum concentration of type I collagen metabolites as a quantitative marker of bone metastases in patients with prostate carcinoma. Cancer 80, 1760–1767 (1997).
Pelger, R. C., Hamdy, N. A., Zwinderman, A. H., Lycklama a Nijeholt, A. A. & Papapoulos, S. E. Effects of the bisphosphonate olpadronate in patients with carcinoma of the prostate metastatic to the skeleton. Bone 22, 403–408 (1998).
Yi, B. et al. PDGF in breast cancer promotes osteosclerotic bone metastases. J. Bone Min. Res. 15 (Suppl 1) A1035 (2000).
Adami, S. Bisphosphonates in prostate carcinoma. Cancer 80, 1674–1679 (1997).
Liotta, L. A. & Kohn, E. C. The microenvironment of the tumour–host interface. Nature 411, 375–379 (2001).An excellent review that cites evidence for the importance of the stroma in cancer-cell behaviour at primary and metastatic sites.
Liotta, L. A. & Kohn, E. Cancer invasion and metastases. JAMA 263, 1123–1126 (1990).
Fidler, I. J. Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res. 38, 2651–2660 (1978).
Zetter, B. R. The cellular basis of site-specific tumor metastasis. N. Engl. J. Med. 322, 605–612 (1990).
Southby, J. et al. Immunohistochemical localization of parathyroid hormone-related protein in breast cancer. Cancer Res. 50, 7710–7716 (1990).
Powell, G. J. et al. Localization of parathyroid hormone-related protein in breast cancer metastases: increased incidence in bone compared with other sites. Cancer Res. 51, 3059–3061 (1991).Clinical data that shows evidence for increased PTHrP expression in bone metastatic sites in patients with breast cancer.
Bryden, A. A., Hoyland, J. A., Freemont, A. J., Clarke, N. W. & George, N. J. Parathyroid hormone related peptide and receptor expression in paired primary prostate cancer and bone metastases. Br. J. Cancer 86, 322–325 (2002).
Miki, T., Yano, S., Hanibuchi, M. & Sone, S. Bone metastasis model with multiorgan dissemination of human small-cell lung cancer (SBC-5) cells in natural killer cell-depleted SCID mice. Oncol. Res. 12, 209–217 (2001).
Henderson, M. et al. Parathyroid hormone-related protein production by breast cancers, improved survival, and reduced bone metastases. J. Natl Cancer Inst. 93, 234–237 (2001).
Guise, T. A. et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast-cancer-mediated osteolysis. J. Clin. Invest. 98, 1544–1549 (1996).Human breast cancer cells, when inoculated into the systemic circulation of nude mice, cause osteolytic lesions that are abrogated by neutralizing antibodies to the tumour peptide PTHrP.
Stewart, A. F. PTHrP (1–36) as a skeletal anabolic agent for the treatment of osteoporosis. Bone 19, 303–306 (1996).
Zhang, Q. X., Borg, A., Wolf, D. M., Oesterreich, S. & Fuqua, S. A. An estrogen receptor mutant with strong hormone-independent activity from a metastatic breast cancer. Cancer Res. 57, 1244–1249 (1997).
De Larco, J. E. et al. A potential role for IL-8 in the metastatic phenotype of breast carcinoma cells. Am. J. Pathol. 158, 639–646 (2001).
Downey, S. E. et al. Expression of the receptor for parathyroid hormone-related protein in normal and malignant breast tissue. J. Pathol. 183, 212–217 (1997).
Gallwitz, W. E. et al. Low doses of 6-thioguanine and 6–mercaptoguanosine inhibit osteolytic bone disease caused by human breast cancer. J. Bone Min. Res. 15 (Suppl. 1), S200 (2000).
Ogata, E. PTHrP, paraneoplastic syndrome and cancer metastasis. J. Bone Min. Metab, 19 (Suppl. 1), S1–S2 (2001).
Zhang, J. et al. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J. Clin. Invest. 107, 1235–1244 (2001).
Nelson, J. B. et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nature Med. 1, 944–949 (1995).
Yin, J. J. et al. Endothelin A receptor blockade inhibits osteoblastic metastases. J. Bone Min. Res. 15 (Suppl. 1), 1254 (2000).
Yin, J. J. et al. Osteoblastic bone metastases: tumor-produced endothelin-1 mediates new bone formation via the endothelin A receptor. J. Back Musculoskeletal. Rehabil. 14 (Suppl. 1), F400 (1999).Data confirming the molecular mechanisms that are responsible for the 'vicious cycle' in breast cancer metastasis to bone.
Marcelli, M. et al. A single nucleotide substitution introduces a premature termination codon into the androgen receptor gene of a patient with receptor-negative androgen resistance. J. Clin. Invest. 85, 1522–1528 (1990).
Marquardt, H., Lioubin, M. N. & Ikeda, T. Complete amino acid sequence of human transforming growth factor type-β2. J. Biol. Chem. 262, 12127–12131 (1987).
Harris, S. E. et al. Effects of transforming growth factor-β on bone nodule formation and expression of bone morphogenic protein 2, osteocalcin, osteopontin, alkaline phosphatase, and type 1 collagen mRNA in long-term cultures of fetal rat calvarial osteoblasts. J. Bone Miner. Res. 9, 855–863 (1994).
Rabbani, S. A. et al. An amino-terminal fragment of urokinase isolated from a prostate cancer cell line (PC-3) is mitogenic for osteoblast-like cells. Biochem. Biophys. Res. Commun. 173, 1058–1064 (1990).
Achbarou, A. et al. Urokinase overproduction results in increased skeletal metastasis by prostate cancer cells in vivo. Cancer Res. 54, 2372–2377 (1994).
Rabbani, S. A. et al. Structural requirements for the growth factor activity of the amino-terminal domain of urokinase. J. Biol. Chem. 267, 14151–14156 (1992).
Dallas, S. L. et al. Characterization and autoregulation of latent TGF-β complexes in osteoblast-like cell lines: production of a latent complex lacking the latent TGF-β-binding protein (LTBP). J. Biol. Chem. 269, 6815–6822 (1994).
Dallas, S. L. et al. Dual role for the latent transforming growth actor-β binding protein in storage of latent TGF-β in the extracellular matrix and as a structural matrix protein. J. Cell Biol. 131, 539–549 (1995).
Cramer, S. D., Chen, Z. & Peehl, D. M. Prostate specific antigen cleaves parathyroid hormone-related protein in the PTH-like domain: inactivation of PTHrP stimulated cAMP accumulation in mouse osteoblasts. J. Urol. 156, 526–531 (1996).
Iwamura, M., Hellman, J., Cockett, A. T., Lilja, H. & Gershagen, S. Alteration of the hormonal bioactivity of parathyroid hormone-related protein (PTHrP) as a result of limited proteolysis by prostate-specific antigen. Urology 48, 317–325 (1996).
Matuo, Y. et al. Heparin binding affinity of rat prostate growth factor in normal and cancerous prostate: partial purification and characterization of rat prostate growth factor in the Dunning tumor. Cancer Res. 47, 188–192 (1987).
Mansson, P. E. et al. HBGF1 gene expression in normal rat prostate and two transplantable rat prostate tumors. Cancer Res. 49, 2485–2494 (1989).
Canalis, E. et al. Effects of endothelial cell growth factor on bone remodeling in vitro. J. Clin. Invest. 79, 52–58 (1987).
Canalis, E., Centrella, M. & McCarthy, T. Effects of basic fibroblast growth factor on bone formation in vitro. J. Clin. Invest. 81, 1572–1577 (1988).
Mayahara, H. et al. In vivo stimulation of endosteal bone formation by basic fibroblast growth factor in rats. Growth Factors 9, 73–80 (1993).
Dunstan, C. R. et al. Systemic administration of acidic fibroblast growth factor (FGF-1) prevents bone loss and increases new bone formation in ovariectomized rats. J. Bone Miner. Res. 14, 953–959 (1999).
Izbicka, E. et al. Human amniotic tumor which induces new bone formation in vivo produces a growth regulatory activity in vitro for osteoblasts identified as an extended form of basic fibroblast growth factor (bFGF). Cancer Res. 56, 633–636 (1996).
Yi, B., Williams, P. J., Niewolna, M., Wang, Y. & Yoneda, T. Tumor-derived PDGF-BB plays a critical role in osteosclerotic bone metastasis in an animal model of human breast cancer. Cancer Res. 62, 917–923 (2002).
Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1, 571–573 (1889).The classical paper describing the 'seed and soil' hypothesis of tumour-cell metastasis.
Yin, J. J. et al. TGF-β signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Invest. 103, 197–206 (1999).
Hiraga, T., Williams, P. J., Mundy, G. R. & Yoneda, T. The bisphosphonate ibandronate promotes apoptosis in MDA-MB-231 human breast cancer cells in bone metastases. Cancer Res. 61, 4418–4424 (2001).
Rasmussen, A. A. & Cullen, K. J. Paracrine/autocrine regulation of breast cancer by the insulin-like growth factors. Breast Cancer Res. Treat. 47, 219–233 (1998).
Yu, H. & Rohan, T. Role of the insulin-like growth factor family in cancer development and progression. J. Natl Cancer Inst. 92, 1472–1489 (2000).
Sasaki, A. et al. Bisphosphonate risedronate reduces metastatic human breast cancer burden in bone in nude mice. Cancer Res. 55, 3551–3557 (1995).In a preclinical model of human breast cancer metastasis to bone, bisphosphonates reduce not only osteolysis but also tumour burden.
Diel, I. J. et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N. Engl. J. Med. 339, 357–363 (1998).The authors show, in a controversial study, that the bisphosphonate clodronate reduces tumour burden in both bone and non-bone metastatic sites in patients with breast cancer.
Powles, T. J. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl Cancer Inst. 91, 730 (1999).
Saarto, T., Blomqvist, C., Virkkunen, P. & Elomaa, I. Adjuvant clodronate treatment does not reduce the frequency of skeletal metastases in node-positive breast cancer patients: 5-year results of a randomized controlled trial. J. Clin. Oncol. 19, 10–17 (2001).
Griepp, P. et al. A single subcutaneous dose of an osteoprotegerin (OPG) construct (AMGN–0007) causes a profound and sustained decrease of bone resorption comparable to standard intravenous bisphosphonate in patients with multiple myeloma. Blood 92 (Suppl. 1), A3227 (1998).
Oyajobi, B. O. et al. Therapeutic efficacy of a soluble receptor activator of nuclear factor-κB–IgG-Fc fusion protein in suppressing bone resorption and hypercalcemia in a model of humoral hypercalcemia of malignancy. Cancer Res. 61, 2572–2578 (2001).
Kuehl, W. M. & Bergsagel, P. L. Multiple myeloma: evolving genetic events and host interactions. Nature Rev. Cancer 2, 175–187 (2002).
Seyberth, H. W. et al. Prostaglandins as mediators of hypercalcemia associated with certain types of cancer. N. Engl. J. Med. 293, 1278–1283 (1975).
Lee, M. V., Fong, E. M., Singer, F. R. & Guenette, R. S. Bisphosphonate treatment inhibits the growth of prostate cancer cells. Cancer Res. 61, 2602–2608 (2001).
Sanders, J. L. et al. Extracellular calcium-sensing receptor expression and its potential role in regulating parathyroid hormone-related peptide secretion in human breast cancer cell lines. Endocrinology 141, 4357–4364 (2000).
Sanders, J. L., Chattopadhyay, N., Kifor, O., Yamaguchi, T. & Brown, E. M. Ca2+-sensing receptor expression and PTHrP secretion in PC-3 human prostate cancer cells. Am. J. Physiol. Endocrinol. Metab. 281, E1267–E1274 (2001).
Moseley, J. M. et al. Parathyroid hormone-related protein purified from a human lung cancer cell line. Proc. Natl Acad. Sci. USA 84, 5048–5052 (1987).
Stewart, A. F., Wu, T., Goumas, D., Burtis, W. J. & Broadus, A. E. N-terminal amino acid sequence of two novel tumor-derived adenylate cyclase-stimulating proteins: identification of parathyroid hormone-like and parathyroid hormone-unlike domains. Biochem. Biophys. Res. Commun. 146, 672–678 (1987).
Strewler, G. J. et al. N-terminal amino acid sequence of two novel tumor-derived adenylate cyclase stimulating proteins: idenfication of parathyroid hormone-unlike domains. Biochem. Biophys. Res. Commun. 146, 672–678 (1987).
Yates, A. J. P. et al. Effects of a synthetic peptide of a parathyroid hormone-related protein on calcium homeostasis, renal tubular calcium reabsorption and bone metabolism. J. Clin. Invest. 81, 932 (1988).
Mundy, G. R. Calcium Homeostasis: Hypercalcemia and Hypocalcemia 2nd edn (Martin Dunitz, London, 1990).
Mundy, G. R., Wilkinson, R. & Heath, D. A. Comparative study of available medical therapy for hypercalcemia of malignancy. Am. J. Med. 74, 421–432 (1983).
Silverman, I. E. & Flynn, J. A. Images in clinical medicine. Ivory vertebra. N. Engl. J. Med. 338, 100 (1998).
Fidler, I. J. Modulation of the organ microenvironment for treatment of cancer metastasis. J. Natl Cancer Inst. 87, 1588–1592 (1995).
Author information
Authors and Affiliations
Related links
Related links
DATABASES
Cancer.gov
LocusLink
Medscape DrugInfo
OMIM
FURTHER INFORMATION
University of Michigan Comprehensive Cancer Center — Bone Metastasis Facts
Glossary
- LEUKOERYTHROBLASTIC ANAEMIA
-
A type of anaemia that is associated with cancers that involve the bone marrow, and that is accompanied by increased production of white blood cells.
- ALKALINE PHOSPHATASE
-
An enzyme that is found on the cell surface of osteoblasts, is involved in bone mineralization and is used as a serum marker of increased osteoblast activity.
- BONE-SCANNING AGENTS
-
Agents, such as bisphosphonates, which localize to bone, that can be tagged with an isotope that allows their detection by imaging techniques. These are used in clinical medicine to detect sites of active bone turnover or bone disease.
- OSTEOCALCIN
-
A protein constituent of bone. Its function remains to be clarified, but circulating levels are used as a marker of osteoblast activity.
- MYELOMA
-
A neoplastic disorder of the plasma cells that is associated with extensive bone destruction and production of large amounts of specific immunoglobulins.
- CALVARIA
-
The skull bones. Rodent calvaria are frequently used in organ culture experiments to determine the effects of factors or compounds that stimulate or inhibit bone resorption or bone formation.
Rights and permissions
About this article
Cite this article
Mundy, G. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2, 584–593 (2002). https://doi.org/10.1038/nrc867
Issue Date:
DOI: https://doi.org/10.1038/nrc867
This article is cited by
-
Prophylactic Radiotherapy Of MInimally Symptomatic Spinal Disease (PROMISSeD): study protocol for a randomized controlled trial
Trials (2024)
-
Aussagekraft von Prokollagen Typ I aminoterminales Propeptid (P1NP) als Knochenaufbaumarker
Zentralblatt für Arbeitsmedizin, Arbeitsschutz und Ergonomie (2024)
-
Microwave ablation combined with vertebral augmentation under real-time temperature monitoring for the treatment of painful spinal osteogenic metastases
BMC Neurology (2023)
-
Mapping lesion-specific response and progression dynamics and inter-organ variability in metastatic colorectal cancer
Nature Communications (2023)
-
New insights into the correlations between circulating tumor cells and target organ metastasis
Signal Transduction and Targeted Therapy (2023)