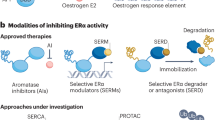
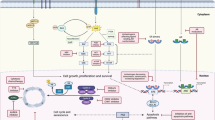
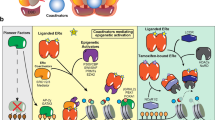
Key Points
-
Breast cancer is the most common cancer of women in the western world. In most cases, breast cancer is oestrogen dependent, and treatment with oestrogen antagonists that inhibit oestrogen receptor (ER) action, particularly tamoxifen, has contributed to a dramatic reduction in breast cancer mortality. However, a substantial proportion of patients presenting with localized disease, and all of the patients with metastatic disease, become resistant to endocrine therapies.
-
In most cases, the ER is present in resistant tumours, and in many of these its activity continues to regulate tumour growth.
-
Resistance to endocrine therapy potentially arises by: ER activation in the absence of oestrogen; hypersensitivity of ER to low levels of circulating oestrogens; or ER activation, rather than inhibition, by oestrogen antagonists.
-
At the molecular level, mechanisms responsible for resistance include:
-
ER mutations that result in increased sensitivity to ligand and/or co-activator recruitment, and a resultant increase in ER activity.
-
Post-translational modifications that result in ligand-independent activation of the ER. These modifications can be triggered by the oncogenic activation of growth-factor signalling pathways.
-
Increased expression of the co-activator proteins that mediate ER activity. By contrast, downregulation of corepressor activity reduces the inhibitory potential of tamoxifen.
-
Mitogenic and anti-apoptotic effects can be mediated by non-genomic effects of the ER, through direct interaction with key components of several signal-transduction pathways. Altered activity of these pathways could contribute to resistance.
-
By understanding which of these pathways could be involved in mediating resistance, we might be able to develop strategies for overcoming or bypassing such resistance.
Abstract
Deaths from breast cancer have fallen markedly over the past decade due, in part, to the use of endocrine agents that reduce the levels of circulating oestrogens or compete with oestrogen for binding to its receptor. However, many breast tumours either fail to respond or become resistant to endocrine therapies. By understanding the mechanisms that underlie this resistance, we might be able to develop strategies for overcoming or bypassing it.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kelsey, J. L. & Berkowitz, G. S. Breast cancer epidemiology. Cancer Res. 48, 5615–5623 (1988).
Beatson, G. T. On the treatment of inoperable cases of carcinoma of the mammary. Suggestions for a new method of treatment with illustrative cases. Lancet 2, 104–107, 162–165 (1896).
Osborne, C. K. Tamoxifen in the treatment of breast cancer. N. Engl. J. Med. 339, 1609–1618 (1998).
MacGregor, J. I. & Jordan, V. C. Basic guide to the mechanisms of antiestrogen action. Pharmacol. Rev. 50, 151–196 (1998).
Buzdar, A. & Howell, A. Advances in aromatase inhibition: clinical efficacy and tolerability in the treatment of breast cancer. Clin. Cancer Res. 7, 2620–2635 (2001).
Russo, J. & Russo, I. H. in The Mammary Gland: Development, Regulation and Function (eds Neville, M. C. & Daniel, C. W.) (Plenum, New York, 1987).
Russo, J. & Russo, I. H. Toward a physiological approach to breast cancer prevention. Cancer Epidemiol. Biomarkers Prev. 3, 353–364 (1994).
Cunha, G. R. et al. Elucidation of a role for stromal steroid hormone receptors in mammary gland growth and development using tissue recombinants. J. Mammary Gland Biol. Neoplasia 2, 393–402 (1997).
Ricketts, D. et al. Estrogen and progesterone receptors in the normal female breast. Cancer Res. 51, 1817–1822 (1991).
Anderson, E., Clarke, R. B. & Howell, A. Estrogen responsiveness and control of normal human breast proliferation. J. Mammary Gland Biol. Neoplasia 3, 23–35 (1998).
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Lakhani, S. R. The transition from hyperplasia to invasive carcinoma of the breast. J. Pathol. 187, 272–278 (1999).
Clarke, R. B., Howell, A., Potten, C. S. & Anderson, E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 57, 4987–4991 (1997).
Russo, J., Ao, X., Grill, C. & Russo, I. H. Pattern of distribution of cells positive for estrogen receptor-α and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res. Treat. 53, 217–227 (1999). References 13 and 14 show that in the normal breast, ER-positive cells are non-proliferative.
Simpson, E. R. & Davis, S. R. Minireview: aromatase and the regulation of estrogen biosynthesis — some new perspectives. Endocrinology 142, 4589–4594 (2001).
Pasqualini, J. R. et al. Concentrations of estrone, estradiol, and estrone sulfate and evaluation of sulfatase and aromatase activities in pre- and postmenopausal breast cancer patients. J. Clin. Endocrinol. Metab. 81, 1460–1464 (1996).
Castagnetta, L. A. et al. Estrogen content and metabolism in human breast tumor tissues and cells. Ann. NY Acad. Sci. 784, 314–324 (1996).
Safe, S. H. Interactions between hormones and chemicals in breast cancer. Annu. Rev. Pharmacol. Toxicol. 38, 121–158 (1998).
Clemons, M. & Goss, P. Estrogen and the risk of breast cancer. N. Engl. J. Med. 344, 276–285 (2001).
Cavalieri, E. L. et al. Molecular origin of cancer: catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc. Natl Acad. Sci. USA 94, 10937–10942 (1997).
Huang, C. S. et al. Breast cancer risk associated with genotype polymorphism of the estrogen-metabolizing genes CYP17, CYP1A1, and COMT: a multigenic study on cancer susceptibility. Cancer Res. 59, 4870–4875 (1999).
Khan, S. A., Rogers, M. A., Khurana, K. K., Meguid, M. M. & Numann, P. J. Estrogen receptor expression in benign breast epithelium and breast cancer risk. J. Natl Cancer Inst. 90, 37–42 (1998). Identifies a link between ER expression in the benign breast and breast cancer risk.
Markopoulos, C., Berger, U., Wilson, P., Gazet, J. C. & Coombes, R. C. Oestrogen receptor content of normal breast cells and breast carcinomas throughout the menstrual cycle. Br Med J 296, 1349–1351 (1988).| PubMed |
Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 351, 1451–1467 (1998).
Klijn, J. G. et al. Combined tamoxifen and luteinizing hormone-releasing hormone (LHRH) agonist versus LHRH agonist alone in premenopausal advanced breast cancer: a meta-analysis of four randomized trials. J. Clin. Oncol. 19, 343–353 (2001).
Coombes, R. C. et al. Idoxifene: report of a phase I study in patients with metastatic breast cancer. Cancer Res. 55, 1070–1074 (1995).
Howell, A. et al. Pharmacokinetics, pharmacological and anti-tumour effects of the specific anti-oestrogen ICI 182780 in women with advanced breast cancer. Br. J. Cancer 74, 300–308 (1996).
Mangelsdorf, D. J. et al. The nuclear receptor superfamily: the second decade. Cell 83, 835–839 (1995).
Schwabe, J. W., Chapman, L., Finch, J. T. & Rhodes, D. The crystal structure of the estrogen receptor DNA-binding domain bound to DNA: how receptors discriminate between their response elements. Cell 75, 567–578 (1993).
Tsai, M. J. & O'Malley, B. W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 63, 451–486 (1994).
Smith, C. L. Cross-talk between peptide growth factor and estrogen receptor signaling pathways. Biol. Reprod. 58, 627–632 (1998).
Gronemeyer, H. Transcription activation by estrogen and progesterone receptors. Annu. Rev. Genet. 25, 89–123 (1991).
Sadovsky, Y. et al. Transcriptional activators differ in their responses to overexpression of TATA-box-binding protein. Mol. Cell. Biol. 15, 1554–1563 (1995).
Ing, N. H., Beekman, J. M., Tsai, S. Y., Tsai, M. J. & OqMalley, B. W. Members of the steroid hormone receptor superfamily interact with TFIIB (S300-II). J. Biol. Chem. 267, 17617–17623 (1992).
Jacq, X. et al. Human TAFII30 is present in a distinct TFIID complex and is required for transcriptional activation by the estrogen receptor. Cell 79, 107–117 (1994).
Glass, C. K. & Rosenfeld, M. G. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 14, 121–141 (2000).
McKenna, N. J. & O'Malley, B. W. From ligand to response: generating diversity in nuclear receptor coregulator function. J. Steroid Biochem. Mol. Biol. 74, 351–356 (2000).
Kingston, R. E. & Narlikar, G. J. ATP-dependent remodeling and acetylation as regulators of chromatin fluidity. Genes Dev. 13, 2339–2352 (1999).
Workman, J. L. & Kingston, R. E. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu. Rev. Biochem. 67, 545–579 (1998).
Sudarsanam, P. & Winston, F. The Swi/Snf family nucleosome-remodeling complexes and transcriptional control. Trends Genet. 16, 345–351 (2000).
Ito, M. & Roeder, R. G. The TRAP/SMCC/mediator complex and thyroid hormone receptor function. Trends Endocrinol. Metab. 12, 127–134 (2001).
Grunstein, M. Histone acetylation in chromatin structure and transcription. Nature 389, 349–352 (1997).
Struhl, K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 12, 599–606 (1998).
Strahl, B. D. & Allis, C. D. The language of covalent histone modifications. Nature 403, 41–45 (2000).
McKenna, N. J., Lanz, R. B. & O'Malley, B. W. Nuclear receptor coregulators: cellular and molecular biology. Endocr. Rev. 20, 321–344 (1999).
Leo, C. & Chen, J. D. The SRC family of nuclear receptor coactivators. Gene 245, 1–11 (2000).
Bannister, A. J. & Kouzarides, T. The CBP co-activator is a histone acetyltransferase. Nature 384, 641–643 (1996).
Ogryzko, V. V., Schiltz, R. L., Russanova, V., Howard, B. H. & Nakatani, Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87, 953–959 (1996).
Spencer, T. E. et al. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature 389, 194–198 (1997).
Chen, H. et al. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 90, 569–580 (1997).
Horlein, A. J. et al. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 377, 397–404 (1995).
Chen, J. D. & Evans, R. M. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 377, 454–457 (1995).
Sande, S. & Privalsky, M. L. Identification of TRACs (T3 receptor-associating cofactors), a family of cofactors that associate with, and modulate the activity of, nuclear hormone receptors. Mol. Endocrinol. 10, 813–825 (1996).
Heinzel, T. et al. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature 387, 43–48 (1997).
Nagy, L. et al. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell 89, 373–380 (1997).
Koh, S. S., Chen, D., Lee, Y. H. & Stallcup, M. R. Synergistic enhancement of nuclear receptor function by p160 coactivators and two coactivators with protein methyltransferase activities. J. Biol. Chem. 276, 1089–1098 (2001).
Chen, D. et al. Regulation of transcription by a protein methyltransferase. Science 284, 2174–2177 (1999).
Stallcup, M. R. Role of protein methylation in chromatin remodeling and transcriptional regulation. Oncogene 20, 3014–3020 (2001).
Torchia, J. et al. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature 387, 677–684 (1997).
Heery, D. M., Kalkhoven, E., Hoare, S. & Parker, M. G. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387, 733–736 (1997).
Shiau, A. K. et al. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 95, 927–937 (1998). References 59 and 60 describe how the L–X–X–L–L-containing peptide mediates co-activator recruitment to the LBD. Reference 61 describes the X-ray crystallographic structure of ER bound by oestrogens and anti-oestrogens. References 69–71 further show that a similar peptide in corepressors is responsible for their interaction with the LBD of nuclear receptors.
Metzger, D., Berry, M., Ali, S. & Chambon, P. Effect of antagonists on DNA binding properties of the human estrogen receptor in vitro and in vivo. Mol. Endocrinol. 9, 579–591 (1995).
Gibson, M. K. et al. The mechanism of ICI 164,384 antiestrogenicity involves rapid loss of estrogen receptor in uterine tissue. Endocrinology 129, 2000–2010 (1991).
Dauvois, S., Danielian, P. S., White, R. & Parker, M. G. Antiestrogen ICI 164,384 reduces cellular estrogen receptor content by increasing its turnover. Proc. Natl Acad. Sci. USA 89, 4037–4041 (1992).
Lerner, L. J. & Jordan, V. C. Development of antiestrogens and their use in breast cancer: eighth Cain memorial award lecture. Cancer Res. 50, 4177–4189 (1990).
Jordan, V. C., MacGregor, J. I. & Tonetti, D. A. Tamoxifen: from breast cancer therapy to the design of a postmenopausal prevention maintenance therapy. Clin. Oncol. 9, 390–394 (1997). | PubMed |
Cosman, F. & Lindsay, R. Selective estrogen receptor modulators: clinical spectrum. Endocr. Rev. 20, 418–434 (1999).
Lavinsky, R. M. et al. Diverse signaling pathways modulate nuclear receptor recruitment of N-CoR and SMRT complexes. Proc. Natl Acad. Sci. USA 95, 2920–2925 (1998). Shows that downregulation of NCOR1 reduces the anti-oestrogenic activity of tamoxifen and might result in resistance.
Hu, X. & Lazar, M. A. The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature 402, 93–96 (1999).
Perissi, V. et al. Molecular determinants of nuclear receptor-corepressor interaction. Genes Dev. 13, 3198–3208 (1999).
Nagy, L. et al. Mechanism of corepressor binding and release from nuclear hormone receptors. Genes Dev. 13, 3209–3216 (1999).
Kuukasjarvi, T., Kononen, J., Helin, H., Holli, K. & Isola, J. Loss of estrogen receptor in recurrent breast cancer is associated with poor response to endocrine therapy. J. Clin. Oncol. 14, 2584–2589 (1996).
Hopp, T. A. & Fuqua, S. A. Estrogen receptor variants. J. Mammary Gland Biol. Neoplasia 3, 73–83 (1998).
Zhang, Q. X., Borg, A., Wolf, D. M., Oesterreich, S. & Fuqua, S. A. An estrogen receptor mutant with strong hormone-independent activity from a metastatic breast cancer. Cancer Res. 57, 1244–1249 (1997). Identifies a tyrosine 537-to-asparagine mutation in breast tumours that results in ligand-independent ER activity.
Weis, K. E., Ekena, K., Thomas, J. A., Lazennec, G. & Katzenellenbogen, B. S. Constitutively active human estrogen receptors containing amino acid substitutions for tyrosine 537 in the receptor protein. Mol. Endocrinol. 10, 1388–1398 (1996).
White, R., Sjoberg, M., Kalkhoven, E. & Parker, M. G. Ligand-independent activation of the oestrogen receptor by mutation of a conserved tyrosine. EMBO J. 16, 1427–1435 (1997).
Arnold, S. F., Obourn, J. D., Jaffe, H. & Notides, A. C. Phosphorylation of the human estrogen receptor on tyrosine 537 in vivo and by src family tyrosine kinases in vitro. Mol. Endocrinol. 9, 24–33 (1995).
Wang, C. et al. Direct acetylation of the estrogen receptor-α hinge region by p300 regulates transactivation and hormone sensitivity. J. Biol. Chem. 276, 18375–18383 (2001).
Fuqua, S. A. et al. A hypersensitive estrogen receptor-α mutation in premalignant breast lesions. Cancer Res. 60, 4026–4029 (2000).
Roodi, N. et al. Estrogen receptor gene analysis in estrogen receptor-positive and receptor-negative primary breast cancer. J. Natl Cancer Inst. 87, 446–451 (1995).
Ignar-Trowbridge, D. M. et al. Coupling of dual signaling pathways: epidermal growth factor action involves the estrogen receptor. Proc. Natl Acad. Sci. USA 89, 4658–4662 (1992).
Curtis, S. W. et al. Physiological coupling of growth factor and steroid receptor signaling pathways: estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. Proc. Natl Acad. Sci. USA 93, 12626–12630 (1996).
Chen, D., Pace, P. E., Coombes, R. C. & Ali, S. Phosphorylation of human estrogen receptor-α by protein kinase A regulates dimerization. Mol. Cell. Biol. 19, 1002–1015 (1999).
Miller, W. R., Elton, R. A., Dixon, J. M., Chetty, U. & Watson, D. M. Cyclic AMP binding proteins and prognosis in breast cancer. Br. J. Cancer 61, 263–266 (1990).
Joel, P. B., Traish, A. M. & Lannigan, D. A. Estradiol-induced phosphorylation of serine 118 in the estrogen receptor is independent of p42/p44 mitogen-activated protein kinase. J. Biol. Chem. 273, 13317–13323 (1998).
Chen, D. et al. Activation of estrogen receptor-α by S118 phosphorylation involves a ligand-dependent interaction with TFIIH and participation of CDK7. Mol. Cell 6, 127–137 (2000).
Kato, S. et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 270, 1491–1494 (1995).
Bunone, G., Briand, P. A., Miksicek, R. J. & Picard, D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 15, 2174–2183 (1996). References 87 and 88 show ER phosphorylation by ERK1/2, which results in ligand-independent activation of ER.
Joel, P. B. et al. pp90rsk1 regulates estrogen receptor-mediated transcription through phosphorylation of Ser-167. Mol. Cell. Biol. 18, 1978–1984 (1998).
Coutts, A. S. & Murphy, L. C. Elevated mitogen-activated protein kinase activity in estrogen-nonresponsive human breast cancer cells. Cancer Res. 58, 4071–4074 (1998).
Shim, W. S. et al. Estradiol hypersensitivity and mitogen-activated protein kinase expression in long-term estrogen deprived human breast cancer cells in vivo. Endocrinology 141, 396–405 (2000).
Sivaraman, V. S., Wang, H., Nuovo, G. J. & Malbon, C. C. Hyperexpression of mitogen-activated protein kinase in human breast cancer. J. Clin. Invest. 99, 1478–1483 (1997). Describes overexpression and/or increased activity of ERK1/2 in breast cancer.
Gee, J. M., Robertson, J. F., Ellis, I. O. & Nicholson, R. I. Phosphorylation of ERK1/2 mitogen-activated protein kinase is associated with poor response to anti-hormonal therapy and decreased patient survival in clinical breast cancer. Int. J. Cancer 95, 247–254 (2001). Describes a correlation between ERK1/2 activity and poor response to endocrine therapy in breast cancer.
Benz, C. C. et al. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res. Treat. 24, 85–95 (1993).
Kurokawa, H. et al. Inhibition of HER2/neu (ERBB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Cancer Res. 60, 5887–5894 (2000).
Nicholson, R. I. et al. Modulation of epidermal growth factor receptor in endocrine-resistant, oestrogen receptor-positive breast cancer. Endocr. Relat. Cancer 8, 175–182 (2001).
Datta, S. R., Brunet, A. & Greenberg, M. E. Cellular survival: a play in three Akts. Genes Dev. 13, 2905–2927 (1999).
Martin, M. B. et al. A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology 141, 4503–4511 (2000).
Campbell, R. A. et al. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J. Biol. Chem. 276, 9817–9824 (2001).
Testa, J. R. & Bellacosa, A. AKT plays a central role in tumorigenesis. Proc. Natl Acad. Sci. USA 98, 10983–10985 (2001).
Li, J. et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast and prostate cancer. Science 275, 1943–1947 (1997).
Gayther, S. A. et al. Mutations truncating the EP300 acetylase in human cancers. Nature Genet. 24, 300–303 (2000).
Anzick, S. L. et al. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277, 965–968 (1997). Identified a gene that is frequently amplified in breast cancer as the p160 co-activator NCOA3 (AIB1).
Bautista, S. et al. In breast cancer, amplification of the steroid receptor coactivator gene AIB1 is correlated with estrogen and progesterone receptor positivity. Clin. Cancer Res. 4, 2925–2929 (1998). Correlates NCOA3 expression with ER and progesterone receptor status.
Smith, C. L., Nawaz, Z. & OqMalley, B. W. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol. Endocrinol. 11, 657–666 (1997).
Webb, P. et al. Estrogen receptor activation function 1 works by binding p160 coactivator proteins. Mol. Endocrinol. 12, 1605–1618 (1998).
Lau, O. D. et al. HATs off: selective synthetic inhibitors of the histone acetyltransferases p300 and PCAF. Mol. Cell 5, 589–595 (2000).
Turlais, F. et al. High-throughput screening for identification of small molecule inhibitors of histone acetyltransferases using scintillating microplates (flashplate). Anal. Biochem. 298, 62–68 (2001).
Chan, C. M., Lykkesfeldt, A. E., Parker, M. G. & Dowsett, M. Expression of nuclear receptor interacting proteins TIF-1, SUG-1, receptor interacting protein 140, and corepressor SMRT in tamoxifen-resistant breast cancer. Clin. Cancer Res. 5, 3460–3467 (1999).
Vigushin, D. M. et al. Trichostatin A is a histone deacetylase inhibitor with potent antitumor activity against breast cancer in vivo. Clin. Cancer Res. 7, 971–976 (2001).
Cohen, L. A. et al. Chemoprevention of carcinogen-induced mammary tumorigenesis by the hybrid polar cytodifferentiation agent, suberanilohydroxamic acid (SAHA). Anticancer Res. 19, 4999–5005 (1999).
Marks, P. A., Rifkind, R. A., Richon, V. M. & Breslow, R. Inhibitors of histone deacetylase are potentially effective anticancer agents. Clin. Cancer Res. 7, 759–760 (2001).
Zwijsen, R. M. et al. CDK-independent activation of estrogen receptor by cyclin D1. Cell 88, 405–415 (1997).
Neuman, E. et al. Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of CDK4. Mol. Cell. Biol. 17, 5338–5347 (1997).
Wang, T. C. et al. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature 369, 669–671 (1994).
Fantl, V., Stamp, G., Andrews, A., Rosewell, I. & Dickson, C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 9, 2364–2372 (1995).
Sicinski, P. et al. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82, 621–630 (1995).
Zwijsen, R. M., Buckle, R. S., Hijmans, E. M., Loomans, C. J. & Bernards, R. Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1. Genes Dev. 12, 3488–3498 (1998).
McMahon, C., Suthiphongchai, T., DiRenzo, J. & Ewen, M. E. P/CAF associates with cyclin D1 and potentiates its activation of the estrogen receptor. Proc. Natl Acad. Sci. USA 96, 5382–5387 (1999).
Peto, R., Boreham, J., Clarke, M., Davies, C. & Beral, V. UK and USA breast cancer deaths down 25% in year 2000 at ages 20–69 years. Lancet 355, 1822–1823 (2000).
Lakhani, S. R. & Ashworth, A. Microarray and histopathological analysis of tumours: the future and the past? Nature Rev. Cancer 1, 151–157 (2001).
Halberstaeder, I. & Hochsman, A. The artificial menopause and cancer of the breast. J. Am. Med. Assoc. 131, 810–816 (1946).
Boyd, S. On oophorectomy in cancer of the breast. Br. Med. J. 2, 1161–1167 (1900).
Lathrop, A. E. C. & Loeb, L. Further investigations on the origins of tumors in mice. III. On the part played by internal secretions in the spontaneous development of tumors. J. Cancer Res. 1, 1–16 (1916).
Allen, E. & Doisy, E. A. An ovarian hormone: preliminary report on its localization, extraction and partial purification and action in test animals. J. Am. Med. Assoc. 81, 819–821 (1923).
Lacassagne, A. Hormonal pathogenesis of adenocarcinoma of the breast. Am. J. Cancer 27, 217–225 (1936). The first paper to raise the possibility that breast cancer could be prevented by the use of oestrogen antagonists.
Lerner, L. J., Holthaus, J. F. & Thompson, C.R. A nonsteroidal estrogen antagonist 1-(p-2-diethylaminoethoxyphenyl)-1-phenyl-2-p-methoxyphenyl-ethanol. Endocrinology 63, 295–318 (1958).
Lerner, L. J. in Non-steroidal Anti-oestrogens: Molecular Pharmacology and Antitumour Activity (eds Sutherland, R. L. & Jordan, V. C.) 1–6 (Sydney Academic Press, Sydney, 1981).
Jensen, E. V. & Jacobon, H. I. Basic guides to the mechanism of estrogen action. Recent Prog. Horm. Res. 18, 387–414 (1962).
Toft, D. & Gorski, J. A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proc. Natl Acad. Sci. USA 55, 1574–1581 (1966).
Gorski, J., Toft, D., Shyamala, G., Smith, D. & Notides, A. Hormone receptors: studies on the interaction of estrogen with the uterus. Recent Prog. Horm. Res. 24, 45–80 (1968).
Jensen, E. V. et al. A two-step mechanism for the interaction of estradiol with rat uterus. Proc. Natl Acad. Sci. USA 59, 632–638 (1968).
Green, S. et al. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature 320, 134–139 (1986).
Harper, M. J. & Walpole, A. L. Contrasting endocrine activities of cis and trans isomers in a series of substituted triphenylethylenes. Nature 212, 87 (1966).
Fisher, B. et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl Cancer Inst. 90, 1371–1388 (1998).
Veronesi, U. et al. Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomised trial among hysterectomised women. Italian Tamoxifen Prevention Study. Lancet 352, 93–97 (1998).
Powles, T. et al. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital Tamoxifen Randomised Chemoprevention Trial. Lancet 352, 98–101 (1998).
Cummings, S. R. et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. J. Am. Med. Assoc. 281, 2189–2197 (1999).
Kuiper, G. G., Enmark, E., Pelto-Huikko, M., Nilsson, S. & Gustafsson, J. A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl Acad. Sci. USA 93, 5925–5930 (1996).
Mosselman, S., Polman, J. & Dijkema, R. ERβ: identification and characterization of a novel human estrogen receptor. FEBS Lett. 392, 49–53 (1996).
Dotzlaw, H., Leygue, E., Watson, P. H. & Murphy, L. C. Estrogen receptor-β messenger RNA expression in human breast tumor biopsies: relationship to steroid receptor status and regulation by progestins. Cancer Res. 59, 529–532 (1999).
Jarvinen, T. A., Pelto-Huikko, M., Holli, K. & Isola, J. Estrogen receptor-β is coexpressed with ERα and PR and associated with nodal status, grade, and proliferation rate in breast cancer. Am. J. Pathol. 156, 29–35 (2000).
Mann, S. et al. Estrogen receptor-β expression in invasive breast cancer. Hum. Pathol. 32, 113–118 (2001).
Roger, P. et al. Decreased expression of estrogen receptor-β protein in proliferative preinvasive mammary tumors. Cancer Res. 61, 2537–2541 (2001).
Ogawa, S. et al. Molecular cloning and characterization of human estrogen receptor betacx: a potential inhibitor of estrogen action in human. Nucleic Acids Res. 26, 3505–3012 (1998).
Saji, S. J. E., Nilsson, S., Rylander, T., Warner, M. & Gustafsson, J. A. Estrogen receptors-α and -β in the rodent mammary gland. Proc. Natl Acad. Sci. USA 97, 337–342 (2000).
Sun, J. et al. Novel ligands that function as selective estrogens or antiestrogens for estrogen receptor-α or estrogen receptor-β. Endocrinology 140, 800–804 (1999).
Meyers, M. J., Sun, J., Carlson, K. E., Katzenellenbogen, B. S. & Katzenellenbogen, J. A. Estrogen receptor subtype-selective ligands: asymmetric synthesis and biological evaluation of cis- and trans-5,11-dialkyl-5,6,11,12-tetrahydrochrysenes. J. Med. Chem. 42, 2456–2468 (1999).
Howe, L. R., Subbaramaiah, K., Brown, A. M. & Dannenberg, A. J. Cyclooxygenase-2: a target for the prevention and treatment of breast cancer. Endocr. Relat. Cancer 8, 97–114 (2001).
Lange, C. A., Richer, J. K. & Horwitz, K. B. Hypothesis: progesterone primes breast cancer cells for cross-talk with proliferative or antiproliferative signals. Mol. Endocrinol. 13, 829–836 (1999).
Shao, D. & Lazar, M. A. Modulating nuclear receptor function: may the phos be with you. J. Clin. Invest. 103, 1617–1618 (1999).
Migliaccio, A. et al. Tyrosine kinase/p21RAS/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 15, 1292–1300 (1996).
Castoria, G. et al. Non-transcriptional action of oestradiol and progestin triggers DNA synthesis. EMBO J. 18, 2500–2510 (1999).
Kousteni, S. et al. Nongenotropic, sex-nonspecific signalling through the estrogen or androgen receptors: dissocation from transcriptional activity. Cell 104, 719–730 (2001).
Simoncini, T. et al. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 407, 538–541 (2000).
Castoria, G. et al. PI3-kinase in concert with Src promotes the S-phase entry of oestradiol-stimulated MCF-7 cells. EMBO J. 20, 6050–6059 (2001).
Paech, K. et al. Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science 277, 1508–1510 (1997).
Acknowledgements
We thank L. Buluwela and C. Palmieri for helpful discussions and M. Slade for ER staining slides. We also thank G. Greene for ER LBD structures. We apologize to those whose work could not be cited due to space constraints.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
FURTHER INFORMATION
Breast Cancer Information Core
Glossary
- INVOLUTION
-
Restoration of the normal size of an organ.
- END BUD
-
Epithelial structures from which the milk-producing alveoli of the mammary gland develop during pregnancy.
- MESENCHYME
-
Embryonic tissue composed of loosely organized, unpolarized cells of both mesodermal and ectodermal (for example, neural crest) origin, with a proteoglycan-rich extracellular matrix.
- OSTEOBLASTS
-
Cells that reside in bone, where they are responsible for depositing the bone matrix.
- CHONDROCYTES
-
Cells that produce cartilage.
- CYTOCHROME P450 ENZYME COMPLEX
-
Haem-containing enzymes that oxidize small molecules, including many carcinogens.
- ADJUVANT THERAPY
-
Therapy given in addition to the primary form of treatment — for example, after surgery.
- MICROMETASTASES
-
Clinically undetectable secondary tumours.
- ZINC FINGER
-
A protein module in which conserved cysteine or histidine residues coordinate a zinc atom. Some zinc-finger regions bind specific DNA sequences; others are involved in protein–protein interactions.
- GENERAL TRANSCRIPTION MACHINERY
-
A set of protein complexes, including RNA polymerase II, that are absolutely required for the initiation of mRNA synthesis and that are sufficient for low-level initiation of mRNA synthesis, at least in vitro.
- HISTONE ACETYLTRANSFERASE
-
An enzyme that catalyses the addition of acetyl groups to lysine residues on the amino-terminal tails of histones. Histone acetylation leads to chromatin decondensation and increased rates of transcriptional initiation.
- HISTONE DEACETYLASE
-
An enzyme that catalyses the removal of acetyl groups from lysine residues on the amino-terminal tails of histones. Histone deacetylation leads to chromatin condensation and decreased rates of transcriptional initiation.
- BASAL TRANSCRIPTION FACTORS
-
A set of protein complexes that associate with RNA polymerase II during the initiation of all mRNA synthesis. Sometimes called general transcription factors.
Rights and permissions
About this article
Cite this article
Ali, S., Coombes, R. Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer 2, 101–112 (2002). https://doi.org/10.1038/nrc721
Issue Date:
DOI: https://doi.org/10.1038/nrc721
This article is cited by
-
The androgen receptor interacts with GATA3 to transcriptionally regulate a luminal epithelial cell phenotype in breast cancer
Genome Biology (2024)
-
Combining lapatinib and palbociclib inhibits cell proliferation and invasion via AKT signaling pathway in endocrine-resistant breast cancer cells
Medical Oncology (2024)
-
Elacestrant demonstrates strong anti-estrogenic activity in PDX models of estrogen-receptor positive endocrine-resistant and fulvestrant-resistant breast cancer
npj Breast Cancer (2022)
-
Computational discovery of novel human LMTK3 inhibitors by high throughput virtual screening using NCI database
Korean Journal of Chemical Engineering (2022)
-
Carbidopa suppresses estrogen receptor-positive breast cancer via AhR-mediated proteasomal degradation of ERα
Investigational New Drugs (2022)