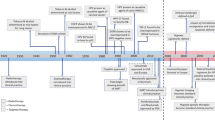
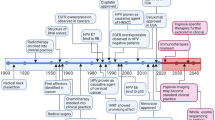
Key Points
-
Head and neck squamous cell carcinomas (HNSCCs) develop in the mucosal linings of the upper aerodigestive tract and are the sixth leading cause of cancer worldwide. Risk factors are exposure to carcinogens, most notably tobacco smoking and alcohol consumption, infection with high-risk types of human papillomavirus (HPV) and genetic predisposition.
-
HNSCC is a heterogeneous disease. At least two genetic subclasses can be distinguished: HPV-positive and HPV-negative tumours. Preliminary data suggest that further subclassification is likely to follow.
-
A key issue in HNSCC pathogenesis is that carcinomas develop within large preneoplastic fields of mucosal epithelium made up of genetically altered cells that are clonally related to the carcinoma and often extend into the surgical margins when tumours are excised, and can cause local recurrences and second primary tumours.
-
Limitless replicative potential of head and neck cancer cells is caused by abrogation of the p53 and retinoblastoma (RB) pathways that perturb cell cycle regulation, probably in the context of telomerase reverse transcriptase (TERT) expression.
-
A subgroup of HNSCCs becomes independent from growth factors owing to somatic changes in the epidermal growth factor receptor (EGFR) signalling pathway.
-
Some, if not all, HNSCCs escape from the growth inhibitory transforming growth factor-β (TGFβ) pathway by somatic mutation or chromosome loss of key genes. This pathway seems to be interconnected to the nuclear factor-κB (NF-κB) pathway.
-
Somatic mutations and genetic changes indicate that the PI3K–PTEN–AKT pathway is frequently activated in HNSCC.
-
Metastatic dissemination of HNSCC is initially to the lymph nodes in the neck. Expression profiles predict lymph node metastasis, but causative cancer genes have not yet been identified.
-
The unravelling of the biological characteristics of HNSCC will lead to novel and personalized therapies in the near future.
Abstract
Head and neck squamous cell carcinomas (HNSCCs) are caused by tobacco and alcohol consumption and by infection with high-risk types of human papillomavirus (HPV). Tumours often develop within preneoplastic fields of genetically altered cells. The persistence of these fields after treatment presents a major challenge, because it might lead to local recurrences and second primary tumours that are responsible for a large proportion of deaths. Aberrant signalling pathways have been identified in HNSCCs and inhibition of epidermal growth factor receptor (EGFR) has proved a successful therapeutic strategy. In this Review, we discuss the recent literature on tumour heterogeneity, field cancerization, molecular pathogenesis and the underlying causative cancer genes that can be exploited for novel and personalized treatments of patients with HNSCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kamangar, F., Dores, G. M. & Anderson, W. F. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 24, 2137–2150 (2006).
Kutler, D. I. et al. High incidence of head and neck squamous cell carcinoma in patients with fanconi anemia. Arch. Otolaryngol. Head Neck Surg. 129, 106–112 (2003).
Hopkins, J. et al. Genetic polymorphisms and head and neck cancer outcomes: a review. Cancer Epidemiol. Biomark. Prev. 17, 490–499 (2008).
Cloos, J. et al. Genetic susceptibility to head and neck squamous cell carcinoma. J. Natl Cancer Inst. 88, 530–535 (1996).
Ang, K. K. et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 363, 24–35 (2010). This paper classifies a subgroup of oropharyngeal carcinoma patients into three classes: poor, intermediate and good prognosis; taking tumour stage, nodal stage, tobacco consumption and HPV status as parameters in the model. The results have important consequences for the design of future clinical trials with survival as an end point.
Smith, R. B., Sniezek, J. C., Weed, D. T. & Wax, M. K. Utilization of free tissue transfer in head and neck surgery. Otolaryngol. Head Neck Surg. 137, 182–191 (2007).
Vergeer, M. R. et al. Intensity-modulated radiotherapy reduces radiation-induced morbidity and improves health-related quality of life: results of a nonrandomized prospective study using a standardized follow-up program. Int. J. Radiat. Oncol. Biol. Phys. 74, 1–8 (2009).
Boscolo-Rizzo, P., Maronato, F., Marchiori, C., Gava, A. & Da Mosto, M. C. Long-term quality of life after total laryngectomy and postoperative radiotherapy versus concurrent chemoradiotherapy for laryngeal preservation. Laryngoscope 118, 300–306 (2008).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000). Defines the acquired phenotypes in cancer cells.
Woolgar, J. A. & Triantafyllou, A. Pitfalls and procedures in the histopathological diagnosis of oral and oropharyngeal squamous cell carcinoma and a review of the role of pathology in prognosis. Oral Oncol. 45, 361–385 (2009).
Chung, C. H. et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell 5, 489–500 (2004). This study generates a classification of HNSCC on the basis of gene expression profiles.
Chung, C. H. et al. Gene expression profiles identify epithelial-to-mesenchymal transition and activation of nuclear factor-kB signalling as characteristic of a high risk squamous cell carcinoma. Cancer Res. 66, 8210–8218 (2006).
Hermsen, M. et al. New chromosomal regions with high-level amplifications in squamous cell carcinomas of the larynx and pharynx, identified by comparative genomic hybridization. J. Pathol. 194, 177–182 (2001).
Jin, C. et al. Cytogenetic abnormalities in 106 oral squamous cell carcinomas. Cancer Genet. Cytogenet. 164, 44–53 (2006).
Smeets, S. J. et al. Genetic classification of oral and oropharyngeal carcinomas identifies subgroups with a different prognosis. Cell. Oncol. 31, 291–300 (2009).
Walboomers, J. M. et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189, 12–19 (1999).
zur Hausen, H. Papillomaviruses and cancer: from basic studies to clinical application. Nature Rev. Cancer 2, 342–350 (2002).
Munoz, N. et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 348, 518–527 (2003).
Syrjanen, S. Human papillomavirus (HPV) in head and neck cancer. J. Clin. Virol. 32, S59–S66 (2005).
Snijders, P. J. F. et al. Prevalence and expression of human papillomavirus in tonsillar carcinomas, indicating a possible viral etiology. Int. J. Cancer 51, 845–850 (1992).
Gillison, M. L. et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl Cancer Inst. 92, 709–720 (2000). This papers shows for the first time that HPV-positive oropharyngeal cancers constitute a distinct clinical disease entity with a markedly improved prognosis compared with HPV-negative cases.
Meijer, C. J. et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int. J. Cancer 124, 516–520 (2009).
Snijders, P. J., van den Brule, A. J. & Meijer, C. J. The clinical relevance of human papillomavirus testing: relationship between analytical and clinical sensitivity. J. Pathol. 201, 1–6 (2003).
Van Houten, V. M. M. et al. Biological evidence that human papillomaviruses are etiologically involved in a subgroup of head and neck squamous cell carcinomas. Int. J. Cancer 93, 232–235 (2001).
Wiest, T., Schwarz, E., Enders, C., Flechtenmacher, C. & Bosch, F. X. Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. Oncogene 21, 1510–1517 (2002).
Braakhuis, B. J. M. et al. Genetic patterns in head and neck cancers that contain or lack transcriptionally active human papillomavirus. J. Natl Cancer Inst. 96, 998–1006 (2004). This paper shows that tumours with transcriptionally active HPV belong to a genetically separate subclass of tumours, typically TP53 wild type. Further data demonstrate the problem of reliable HPV detection.
Smeets, S. J. et al. Genome-wide DNA copy number alterations in head and neck squamous cell carcinomas with or without oncogene-expressing human papillomavirus. Oncogene 25, 2558–2564 (2006).
Slebos, R. J. C. et al. Gene expression differences associated with human papillomavirus status in head and neck squamous cell carcinoma. Clin. Cancer Res. 12, 701–709 (2006).
Smeets, S. J. et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int. J. Cancer 121, 2465–2472 (2007).
Robinson, M., Sloan, P. & Shaw, R. Refining the diagnosis of oropharyngeal squamous cell carcinoma using human papillomavirus testing. Oral Oncol. 46, 492–496 (2010).
D'Souza, G. et al. Case-control study of human papillomavirus and oropharyngeal cancer. N. Engl. J. Med. 356, 1944–1956 (2007). A case–control study showing that an individual's number of sexual partners and oral sex partners is associated with HPV-positive oropharyngeal carcinoma risk.
Ragin, C. C. R. & Taioli, E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int. J. Cancer 121, 1813–1820 (2007).
Poeta, M. L. et al. Tp53 mutations and survival in squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 357, 2552–2561 (2007). This study shows that the type of TP53 mutation has consequences for the prognosis of HNSCC.
Westra, W. H. et al. Inverse relationship between human papillomavirus-16 infection and disruptive p53 gene mutations in squamous cell carcinoma of the head and neck. Clin. Cancer Res. 14, 366–369 (2008).
Napier, S. S. & Speight, P. M. Natural history of potentially malignant oral lesions and conditions: an overview of the literature. J. Oral Pathol. Med. 37, 1–10 (2008).
van der Waal, I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol. 45, 317–323 (2009).
Lodi, G., Sardella, A., Bez, C., Demarosi, F. & Carrassi, A. Interventions for treating oral leukoplakia. Cochrane Database Syst. Rev. CD001829 (2006).
Partridge, M. et al. A case-control study confirms that microsatellite assay can identify patients at risk of developing oral squamous cell carcinoma within a field of cancerization. Cancer Res. 60, 3893–3898 (2000).
Wrangle, J. M. & Khuri, F. R. Chemoprevention of squamous cell carcinoma of the head and neck. Curr. Opinion Oncol. 19, 180–187 (2007).
Schaaij-Visser, T. B. M. et al. Evaluation of cornulin, keratin 4, keratin 13 expression and grade of dysplasia for predicting malignant progression of oral leukoplakia. Oral Oncol. 46, 123–127 (2010).
Rosin, M. P. et al. Use of allelic loss to predict malignant risk for low-grade oral epithelial dysplasia. Clin. Cancer Res. 6, 357–362 (2000).
Mao, L. et al. Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nature Med. 2, 682–685 (1996).
Torres-Rendon, A., Stewart, R., Craig, G. T., Wells, M. & Speight, P. M. DNA ploidy analysis by image cytometry helps to identify oral epithelial dysplasias with a high risk of malignant progression. Oral Oncol. 45, 468–473 (2009).
Shpitzer, T. et al. Salivary analysis of oral cancer biomarkers. Brit. J. Cancer 101, 1194–1198 (2009).
Bremmer, J. F. et al. A noninvasive genetic screening test to detect oral preneoplastic lesions. Lab. Invest. 85, 1481–1488 (2005).
Bremmer, J. F. et al. Screening for oral precancer with noninvasive genetic cytology. Cancer Prev. Res. 2, 128–133 (2009).
Slaughter, D. P., Southwick, H. W. & Smejkal, W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 6, 963–968 (1953). This paper introduces the term 'field cancerization' and links it to the recurrence of oral cancer and second primary tumours.
Califano, J. et al. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res. 56, 2488–2492 (1996). The authors of this paper propose a multi-step carcinogenesis model with a genetic basis for HNSCC.
Tabor, M. P. et al. Persistence of genetically altered fields in head and neck cancer patients: Biological and clinical implications. Clin. Cancer Res. 7, 1523–1532 (2001). This paper demonstrates the importance of fields defined by genetic markers as potential sources of local recurrences and second primary tumours
Tabor, M. P. et al. Comparative molecular and histological grading of epithelial dysplasia of the oral cavity and the oropharynx. J. Pathol. 199, 354–360 (2003).
Willis, R. A. The mode of origin of tumors. Solitary localized squamous cell growths of the skin. Cancer Res. 4, 469–479 (1944).
Slaughter, D. P. Multicentric origin of intraoral carcinoma. Surgery 133–146 (1946).
Hittelman, W. N. Genetic instability in epithelial tissues at risk for cancer. Ann. NY Acad. Sci. 952, 1–12 (2001).
Tabor, M. P. et al. Genetically altered fields as origin of locally recurrent head and neck cancer: a retrospective study. Clin. Cancer Res. 10, 3607–3613 (2004).
Roesch-Ely, M. et al. Proteomic analysis reveals successive aberrations in protein expression from healthy mucosa to invasive head and neck cancer. Oncogene 26, 54–64 (2007).
Schaaij-Visser, T. B. M. et al. Differential proteomics identifies protein biomarkers that predict local relapse of head and neck squamous cell carcinomas. Clin. Cancer Res. 15, 7666–7675 (2009).
Braakhuis, B. J. M., Tabor, M. P., Kummer, J. A., Leemans, C. R. & Brakenhoff, R. H. A genetic explanation of Slaughter's concept of field cancerization: evidence and clinical implications. Cancer Res. 63, 1727–1730 (2003). This paper presents the patch–field–tumour model and shows the clinical relevance of these fields for local recurrences and second primary tumours.
van Houten, V. M. et al. Mutated p53 as a molecular marker for the diagnosis of head and neck cancer. J. Pathol. 198, 476–486 (2002).
Jonason, A. S. et al. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc. Natl Acad. Sci. USA 93, 14025–14029 (1996).
Dakubo, G. D., Jakupciak, J. P., Birch-Machin, M. A. & Parr, R. L. Clinical implications and utility of field cancerization. Cancer Cell. Int. 7, 2 (2007).
Negrini, S., Gorgoulis, V. G. & Halazonetis, T. D. Genomic instability — an evolving hallmark of cancer. Nature Rev. Mol. Cell Biol. 11, 220–228 (2010).
Patmore, H. S., Cawkwell, L., Stafford, N. D. & Greenman, J. Unraveling the chromosomal aberrations of head and neck squamous cell carcinoma: a review. Ann. Surg. Oncol. 12, 831–842 (2005).
Wreesmann, V. B. & Singh, B. Chromosomal aberrations in squamous cell carcinomas of the upper aerodigestive tract: biologic insights and clinical opportunities. J. Oral Pathol. Med. 34, 449–459 (2005).
Ha, P. K., Chang, S. S., Glazer, C. A., Califano, J. A. & Sidransky, D. Molecular techniques and genetic alterations in head and neck cancer. Oral Oncol. 45, 335–339 (2009).
Carbone, M., Klein, G., Gruber, J. & Wong, M. Modern criteria to establish human cancer etiology. Cancer Res. 64, 5518–5524 (2004).
Kastan, M. B. & Bartek, J. Cell-cycle checkpoints and cancer. Nature 432, 316–323 (2004).
Balz, V. et al. Is the p53 inactivation frequency in squamous cell carcinomas of the head and neck underestimated? Analysis of p53 exons 2–11 and human papillomavirus 16/18 E6 transcripts in 123 unselected tumor specimens. Cancer Res. 63, 1188–1191 (2003).
Opitz, O. G. et al. Cyclin D1 overexpression and p53 inactivation immortalize primary oral keratinocytes by a telomerase-independent mechanism. J. Clin. Invest. 108, 725–732 (2001).
Rheinwald, J. G. et al. A two-stage, p16INK4A- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol. Cell. Biol. 22, 5157–5172 (2002).
Smeets, S. J. et al. Immortalization of oral keratinocytes by functional inactivation of the p53 and pRb pathways. Int. J. Cancer (in the press). References 68–70 demonstrate the crucial role of the p53 and Rb pathways in head and neck carcinogenesis.
Reed, A. L. et al. High frequency of p16 (CDKN2/MTS-1/INK4A) inactivation in head and neck squamous cell carcinoma. Cancer Res. 56, 3630–3633 (1996).
Gibcus, J. H. et al. Amplicon mapping and expression profiling identify the Fas-associated death domain gene as a new driver in the 11q13.3 amplicon in laryngeal/pharyngeal cancer. Clin. Cancer Res. 13, 6257–6266 (2007).
Berns, K. et al. A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature 428, 431–437 (2004).
Dickson, M. A. et al. Human keratinocytes that express hTERT and also bypass a p16INK4A-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 20, 1436–1447 (2000).
Snijders, P. J. F. et al. Telomerase activity exclusively in cervical carcinomas and a subset of cervical intraepithelial neoplasia grade III lesions: strong association with elevated messenger RNA levels of its catalytic subunit and high-risk human papillomavirus DNA. Cancer Res. 58, 3812–3818 (1998).
Hynes, N. E. & Lane, H. A. ERBB receptors and cancer: the complexity of targeted inhibitors. Nature Rev. Cancer 5, 341–354 (2005).
Lin, S. Y. et al. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nature Cell Biol. 3, 802–808 (2001).
Lo, H. W. et al. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell 7, 575–589 (2005).
Goessel, G. et al. Creating oral squamous cancer cells: a cellular model of oral–esophageal carcinogenesis. Proc. Natl Acad. Sci. USA 102, 15599–15604 (2005).
Ozanne, B., Richards, C. S., Hendler, F., Burns, D. & Gusterson, B. Over-expression of the EGF receptor is a hallmark of squamous cell carcinomas. J. Pathol. 149, 9–14 (1986).
Grandis, J. R. & Tweardy, D. J. Elevated levels of transforming growth factor α and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 53, 3579–3584 (1993).
Hama, T. et al. Prognostic significance of epidermal growth factor receptor phosphorylation and mutation in head and neck squamous cell carcinoma. Oncologist 14, 900–908 (2009).
Bonner, J. A. et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 354, 567–578 (2006). This study shows that the addition of erbitux, an inhibitor of the EGFR pathway, to radiotherapy leads to clinical benefit for patients with HNSCC.
Morandell, S. et al. Phosphoproteomics strategies for the functional analysis of signal transduction. Proteomics 6, 4047–4056 (2006).
Lee, J. W. et al. Somatic mutations of EGFR gene in squamous cell carcinoma of the head and neck. Clin. Cancer Res. 11, 2879–2882 (2005).
Loeffler-Ragg, J. et al. Low incidence of mutations in EGFR kinase domain in Caucasian patients with head and neck squamous cell carcinoma. Eur. J. Cancer 42, 109–111 (2006).
Ekstrand, A. J., Sugawa, N., James, C. D. & Collins, V. P. Amplified and rearranged epidermal growth factor receptor genes in human glioblastomas reveal deletions of sequences encoding portions of the N- and/or C-terminal tails. Proc. Natl Acad. Sci. USA 89, 4309–4313 (1992).
Sok, J. C. et al. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin. Cancer Res. 12, 5064–5073 (2006).
Ishitoya, J. et al. Gene amplification and overexpression of EGF receptor in squamous cell carcinomas of the head and neck. Brit. J. Cancer 59, 559–562 (1989).
Temam, S. et al. Epidermal growth factor receptor copy number alterations correlate with poor clinical outcome in patients with head and neck squamous cancer. J. Clin. Oncol. 25, 2164–2170 (2007).
Sheu, J. J. C. et al. Functional genomic analysis identified epidermal growth factor receptor activation as the most common genetic event in oral squamous cell carcinoma. Cancer Res. 69, 2568–2576 (2009). This study shows that EGFR amplification is found in 30% of HNSCCs, and coincides with overexpression.
Knudsen, B. S. & Vande Woude, G. Showering c-MET-dependent cancers with drugs. Curr. Opin. Genet. Dev. 18, 87–96 (2008).
Seiwert, T. Y. et al. The MET receptor tyrosine kinase is a potential novel therapeutic target for head and neck squamous cell carcinoma. Cancer Res. 69, 3021–3031 (2009).
Knowles, L. M. et al. HGF and c-Met participate in paracrine tumorigenic pathways in head and neck squamous cell cancer. Clin. Cancer Res. 15, 3740–3750 (2009).
Wang, D. et al. Mutation and downregulation of the transforming growth factor beta type II receptor gene in primary squamous cell carcinomas of the head and neck. Carcinogenesis 18, 2285–2290 (1997).
Huntley, S. P. et al. Attenuated type II TGF-B receptor signalling in human malignant oral keratinocytes induces a less differentiated and more aggressive phenotype that is associated with metastatic dissemination. Int. J. Cancer 110, 170–176 (2004).
Qiu, W., Schonleben, F., Li, X. & Su, G. H. Disruption of transforming growth factor β–Smad signaling pathway in head and neck squamous cell carcinoma as evidenced by mutations of SMAD2 and SMAD4. Cancer Lett. 245, 163–170 (2007).
Bornstein, S. et al. Smad4 loss in mice causes spontaneous head and neck cancer with increased genomic instability and inflammation. J. Clin. Invest. 119, 3408–3419 (2009).
Mishra, A., Bharti, A. C., Varghese, P., Saluja, D. & Das, B. C. Differential expression and activation of NF-κB family proteins during oral carcinogenesis: role of high risk human papillomavirus infection. Int. J. Cancer 119, 2840–2850 (2006).
Karin, M. Nuclear factor-κB in cancer development and progression. Nature 441, 431–436 (2006).
Perkins, N. D. Integrating cell-signalling pathways with NF-κB and IKK function. Nature Rev. Mol. Cell Biol. 8, 49–62 (2007).
Cohen, J. et al. Attenuated transforming growth factor β signaling promotes nuclear factor-κB activation in head and neck cancer. Cancer Res. 69, 3415–3424 (2009).
Engelman, J. A. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nature Rev. Cancer 9, 550–562 (2009).
Kozaki, K. et al. PIK3CA mutation is an oncogenic aberration at advanced stages of oral squamous cell carcinoma. Cancer Sci. 97, 1351–1358 (2006).
Qiu, W. L. et al. PIK3CA mutations in head and neck squamous cell carcinoma. Clin. Cancer Res. 12, 1441–1446 (2006).
Murugan, A. K., Hong, N. T., Fukui, Y., Munirajan, A. K. & Tsuchida, N. Oncogenic mutations of the PIK3CA gene in head and neck squamous cell carcinomas. Int. J. Oncol. 32, 101–111 (2008).
Okami, K. et al. Analysis of PTEN/MMAC1 alterations in aerodigestive tract tumors. Cancer Res. 58, 509–511 (1998).
Redon, R. et al. A simple specific pattern of chromosomal aberrations at early stages of head and neck squamous cell carcinomas: PIK3CA but not p63 gene as a likely target of 3q26-qter gains. Cancer Res. 61, 4122–4129 (2001).
Woenckhaus, J. et al. Genomic gain of PIK3CA and increased expression of p110alpha are associated with progression of dysplasia into invasive squamous cell carcinoma. J. Pathol. 198, 335–342 (2002).
Pantel, K. & Brakenhoff, R. H. Dissecting the metastatic cascade. Nature Rev. Cancer 4, 448–456 (2004).
Rosenthal, E. L. & Matrisian, L. M. Matrix metalloproteases in head and neck cancer. Head Neck 28, 639–648 (2006).
Sun, P. C. et al. Transcript map of the 8p23 putative tumor suppressor region. Genomics 75, 17–25 (2001).
Scholnick, S. B. et al. Chromosome 8 allelic loss and the outcome of patients with squamous cell carcinoma of the supraglottic larynx. J. Natl Cancer Inst. 88, 1676–1682 (1996).
Sunwoo, J. B. et al. Localization of a putative tumor suppressor gene in the sub-telomeric region of chromosome 8p. Oncogene 18, 2651–2655 (1999).
Kraus, D. M. et al. CSMD1 is a novel multiple domain complement-regulatory protein highly expressed in the central nervous system and epithelial tissues. J. Immunol. 176, 4419–4430 (2006).
Roepman, P. et al. An expression profile for diagnosis of lymph node metastases from primary head and neck squamous cell carcinomas. Nature Genet. 37, 182–186 (2005). This study identifies an expression-array profile with predictive value for the development of lymph node metastases.
Thiery, J. P. Epithelial–mesenchymal transitions in tumour progression. Nature Rev. Cancer 2, 442–454 (2002).
Ikushima, H. & Miyazono, K. TGFβ signalling: a complex web in cancer progression. Nature Rev. Cancer 10, 415–424 (2010).
Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 29, 15–18 (2002).
Kerbel, R. S. Tumor angiogenesis. N. Engl. J. Med. 358, 2039–2049 (2008).
Kyzas, P. A., Cunha, I. W. & Ioannidis, J. P. A. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in head and neck squamous cell carcinoma: a meta-analysis. Clin. Cancer Res. 11, 1434–1440 (2005).
Fei, J. et al. Prognostic significance of vascular endothelial growth factor in squamous cell carcinomas of the tonsil in relation to human papillomavirus status and epidermal growth factor receptor. Ann. Surg. Oncol. 16, 2908–2917 (2009).
Virgilio, L. et al. FHIT gene alterations in head and neck squamous cell carcinomas. Proc. Natl Acad. Sci. USA 93, 9770–9775 (1996).
Hibi, K. et al. AIS is an oncogene amplified in squamous cell carcinoma. Proc. Natl Acad. Sci. USA 97, 5462–5467 (2000).
Rocco, J. W., Leong, C. O., Kuperwasser, N., DeYoung, M. P. & Ellisen, L. W. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell 9, 45–56 (2006).
Rodrigo, J. P., Lazo, P. S., Ramos, S., Alvarez, I. & Suarez, C. MYC amplification in squamous cell carcinomas of the head and neck. Arch. Otolaryngol. Head Neck Surg. 122, 504–507 (1996).
Carvalho, A. L. et al. Deleted in colorectal cancer is a putative conditional tumor-suppressor gene inactivated by promoter hypermethylation in head and neck squamous cell carcinoma. Cancer Res. 66, 9401–9407 (2006).
Avissar, M., Christensen, B. C., Kelsey, K. T. & Marsit, C. J. MicroRNA expression ratio is predictive of head and neck squamous cell carcinoma. Clin. Cancer Res. 15, 2850–2855 (2009).
Childs, G. et al. Low-level expression of microRNAs let-7d and miR-205 are prognostic markers of head and neck squamous cell carcinoma. Am. J. Pathol. 174, 736–745 (2009).
Cervigne, N. K. et al. Identification of a microRNA signature associated with progression of leukoplakia to oral carcinoma. Hum. Mol. Genet. 18, 4818–4829 (2009).
Kies, M. S. et al. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. J. Clin. Oncol. 28, 8–14 (2010).
Almadori, G. et al. Multistep laryngeal carcinogenesis helps our understanding of the field cancerisation phenomenon: a review. Eur. J. Cancer 40, 2383–2388 (2004).
Poh, C. F. et al. Fluorescence visualization detection of field alterations in tumor margins of oral cancer patients. Clin. Cancer Res. 12, 6716–6722 (2006).
Roblyer, D. et al. Objective detection and delineation of oral neoplasia using autofluorescence imaging. Cancer Prev. Res. 2, 423–431 (2009). References 133 and 134 show that fluorescence visualization can identify subclinical high-risk fields with cancerous and precancerous changes.
Braakhuis, B. J. M. et al. Second primary tumors and field cancerization in oral and oropharyngeal cancer: molecular techniques provide new insights and definitions. Head Neck 24, 198–206 (2002).
Graveland, A. P. et al. Loss of heterozygosity at 9p and p53 immunopositivity in surgical margins predict local relapse in head and neck squamous cell carcinoma. Int. J. Cancer (in the press).
Derynck, R., Akhurst, R. J. & Balmain, A. TGF-β signaling in tumor suppression and cancer progression. Nature Genet. 29, 117–129 (2001).
Chaturvedi, A. K., Engels, E. A., Anderson, W. F. & Gillison, M. L. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J. Clin. Oncol. 26, 612–619 (2008).
Nasman, A. et al. Incidence of human papilloma virus positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int. J. Cancer 125, 362–366 (2009).
Jung, A. C. et al. Biological and clinical relevance of transcriptionally active human papillomavirus (HPV) infection in oropharynx squamous cell carcinoma. Int. J. Cancer 126, 1882–1894 (2010).
Herrero, R. et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J. Natl Cancer Inst. 95, 1772–1783 (2003).
Hafkamp, H. C. et al. Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int. J. Cancer 122, 2656–2664 (2008).
Hogg, R. P. et al. Frequent 3p allele loss and epigenetic inactivation of the RASSF1A tumour suppressor gene from region 3p21.3 in head and neck squamous cell carcinoma. Eur. J. Cancer 38, 1585–1592 (2002).
Veeriah, S. et al. The tyrosine phosphatase PTPRD is a tumor suppressor that is frequently inactivated and mutated in glioblastoma and other human cancers. Proc. Natl Acad. Sci. USA 106, 9435–9440 (2009).
Somers, K. D. et al. Frequent p53 mutations in head and neck cancer. Cancer Res. 52, 5997–6000 (1992).
Brennan, J. A. et al. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 332, 429–435 (1995).
Katoh, M. & Katoh, M. Identification and characterization of human TIPARP gene within the CCNL amplicon at human chromosome 3q25.31. Int. J. Oncol. 23, 541–547 (2003).
Sarkaria, I. et al. Squamous cell carcinoma related oncogene/DCUN1D1 is highly conserved and activated by amplification in squamous cell carcinomas. Cancer Res. 66, 9437–9444 (2006).
Bockmuhl, U. et al. Patterns of chromosomal alterations in metastasizing and nonmetastasizing primary head and neck carcinomas. Cancer Res. 57, 5213–5216 (1997).
Agochiya, M. et al. Increased dosage and amplification of the focal adhesion kinase gene in human cancer cells. Oncogene 18, 5646–5653 (1999).
Inaba, T. et al. Genomic organization, chromosomal localization, and independent expression of human cyclin D genes. Genomics 13, 565–574 (1992).
Schuuring, E., Verhoeven, E., Mooi, W. J. & Michalides, R. J. Identification and cloning of two overexpressed genes, U21B31/PRAD1 and EMS1, within the amplified chromosome 11q13 region in human carcinomas. Oncogene 7, 355–361 (1992).
Snijders, A. M. et al. Rare amplicons implicate frequent deregulation of cell fate specification pathways in oral squamous cell carcinoma. Oncogene 24, 4232–4242 (2005).
Beder, L. B. et al. Genome-wide analyses on loss of heterozygosity in head and neck squamous cell carcinomas. Lab. Invest. 83, 99–105 (2003).
Acknowledgements
The authors' research summarized here is supported by the Cancer Center Amsterdam/VU Research Institute on Cancer and Immunology, the Dutch Cancer Society, the European Commission (6th framework program), The Netherlands Organization for Scientific Research (NWO), The Fanconi Anaemia Research Fund, The German Fanconi Support Group, the Dutch Children Cancer-free Foundation, and the Center for Translational Molecular Medicine (AIRFORCE project). The authors would like to apologize to those authors whose work could not be cited directly owing to space constraints.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Chemoradiation
-
Combined treatment with chemotherapy (usually cisplatin) and radiation.
- Ploidy
-
The number of chromosomes in a cell. Normal human cells are diploid, having a DNA index of 2c, a state also referred to as euploid. Cancer cells are often tetraploid, with a DNA index of 4c, or aneuploid, with a DNA index somewhere between 2c and 4c. The DNA index reflects the number of numerical genetic changes: the losses and gains of chromosomes or parts of chromosomes.
- Comparative genomic hybridization
-
(CGH). A method to visualize the presence or absence of chromosomes or parts of chromosomes in a tumour sample by fluorescence microscopy. Array CGH is comparable to CGH, except that the labelled DNAs are not hybridized to metaphase spreads but to DNA molecules on a glass slide, which increases the resolution.
- Oral leukoplakia
-
The most common premalignant lesion of HNSCC, defined as a white plaque in the mucosal linings that indicates questionable risk after the exclusion of other known diseases or disorders that carry no increased risk of cancer.
- Squamous epithelium
-
Multilayered epithelium covering the linings of the upper aerodigestive tract.
- Loss of heterozygosity
-
(LOH). A genetic change that describes the loss of one allele of a gene for which the other allele is already inactivated.
- Telomere
-
The linear end of a chromosome. The telomere is shortened with each round of DNA replication.
- MicroRNA
-
(miRNA). Small RNA (of 22–24 oligonucleotides) generated from larger transcripts that bind target sequences in messenger RNAs in a large complex called the RNA-induced silencing complex (RISC). Binding of miRNAs to their (usually multiple) target transcripts causes transcript degradation or inhibition of protein translation.
Rights and permissions
About this article
Cite this article
Leemans, C., Braakhuis, B. & Brakenhoff, R. The molecular biology of head and neck cancer. Nat Rev Cancer 11, 9–22 (2011). https://doi.org/10.1038/nrc2982
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc2982
This article is cited by
-
Prospective observational study of surgery alone for locally advanced oral squamous cell carcinoma: a real-world study
BMC Oral Health (2024)
-
B cells in head and neck squamous cell carcinoma: current opinion and novel therapy
Cancer Cell International (2024)
-
Tumor mutational burden predictability in head and neck squamous cell carcinoma patients treated with immunotherapy: systematic review and meta-analysis
Journal of Translational Medicine (2024)
-
EGFR-Activated JAK2/STAT3 Pathway Confers Neuroprotection in Spinal Cord Ischemia–Reperfusion Injury: Evidence from High-Throughput Sequencing and Experimental Models
Molecular Neurobiology (2024)
-
A stepwise-responsive editor integrated with three copper ions for the treatment of oral squamous cell carcinoma
Nano Research (2024)