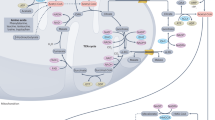
Key Points
-
Acetate can be used by tumour cells as an important bioenergetic fuel or as a nutritional source to support lipid biosynthesis.
-
Acetate can be a precursor for acetylation of histones and other proteins and hence can serve as an epigenetic and post-translational modifier.
-
Despite the low circulating concentration of acetate, acetate may still exert its effects through intra- and intercellular recycling of acetate molecules within the tumour microenvironment or at sites with high local acetate concentrations.
-
Acetyl-CoA synthetase 2 (ACSS2) is emerging as an important enzyme in the growth of many different types of cancers and is induced by cancer cells in response to nutrient stress conditions. Targeting ACSS2 may prove to be a powerful therapeutic modality.
-
ACSS2 is highly upregulated in multiple cancer types and may be a useful biomarker for anti-ACSS2 therapies.
-
[11C]acetate has demonstrated excellent potential as an alternative positron emission tomography imaging probe for cancer. More studies on why some but not other tumours are [11C]acetate avid, and correlating these data with the expression of acetyl-CoA synthetases will aid in deciphering the exact manner by which acetate metabolism supports tumour growth and help in stratifying patients for future anti-ACSS2 therapy.
Abstract
Recent high-profile reports have reignited an interest in acetate metabolism in cancer. Acetyl-CoA synthetases that catalyse the conversion of acetate to acetyl-CoA have now been implicated in the growth of hepatocellular carcinoma, glioblastoma, breast cancer and prostate cancer. In this Review, we discuss how acetate functions as a nutritional source for tumours and as a regulator of cancer cell stress, and how preventing its (re)capture by cancer cells may provide an opportunity for therapeutic intervention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Medes, G., Friedmann, B. & Weinhouse, S. Fatty acid metabolism. VIII. Acetate metabolism in vitro during hepatocarcinogenesis by p-dimethylaminoazobenzene. Cancer Res. 16, 57–62 (1956).
Medes, G. & Weinhouse, S. Metabolism of neoplastic tissue. XIII. Substrate competition in fatty acid oxidation in ascites tumor cells. Cancer Res. 18, 352–359 (1958).
Medes, G., Paden, G. & Weinhouse, S. Metabolism of neoplastic tissues. XI. Absorption and oxidation of dietary fatty acids by implanted tumors. Cancer Res. 17, 127–133 (1957).
Thomlinson, R. H. & Gray, L. H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br. J. Cancer 9, 539–549 (1955).
Nath, A. & Chan, C. Genetic alterations in fatty acid transport and metabolism genes are associated with metastatic progression and poor prognosis of human cancers. Sci. Rep. 6, 18669 (2016).
Zaugg, K. et al. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev. 25, 1041–1051 (2011).
Gajewski, T. F., Schreiber, H. & Fu, Y. X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 14, 1014–1022 (2013).
Azzi, S., Hebda, J. K. & Gavard, J. Vascular permeability and drug delivery in cancers. Front. Oncol. 3, 211 (2013).
Joint FAO/WHO Codex Alimentarius Commission. Codex general standard for food additives http://www.fao.org/fao-who-codexalimentarius/download/standards/4/CXS_192_2015e.pdf (Joint FAO/WHO Codex Alimentarius Commission, 2015).
Suematsu, N. & Isohashi, F. Molecular cloning and functional expression of human cytosolic acetyl-CoA hydrolase. Acta Biochim. Pol. 53, 553–561 (2006).
Horibata, Y., Ando, H., Itoh, M. & Sugimoto, H. Enzymatic and transcriptional regulation of the cytoplasmic acetyl-CoA hydrolase ACOT12. J. Lipid Res. 54, 2049–2059 (2013).
Scheppach, W., Pomare, E. W., Elia, M. & Cummings, J. H. The contribution of the large intestine to blood acetate in man. Clin. Sci. (Lond.) 80, 177–182 (1991).
Louis, P., Hold, G. L. & Flint, H. J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 12, 661–672 (2014).
Cummings, J. H., Pomare, E. W., Branch, W. J., Naylor, C. P. & Macfarlane, G. T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28, 1221–1227 (1987).
Schuchmann, K. & Muller, V. Autotrophy at the thermodynamic limit of life: a model for energy conservation in acetogenic bacteria. Nat. Rev. Microbiol. 12, 809–821 (2014).
Rey, F. E. et al. Dissecting the in vivo metabolic potential of two human gut acetogens. J. Biol. Chem. 285, 22082–22090 (2010).
Donohoe, D. R. et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 13, 517–526 (2011).
Bloemen, J. G. et al. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin. Nutr. 28, 657–661 (2009).
Rocco, A., Compare, D., Angrisani, D., Sanduzzi Zamparelli, M. & Nardone, G. Alcoholic disease: liver and beyond. World J. Gastroenterol. 20, 14652–14659 (2014).
Nuutinen, H., Lindros, K., Hekali, P. & Salaspuro, M. Elevated blood acetate as indicator of fast ethanol elimination in chronic alcoholics. Alcohol 2, 623–626 (1985).
Lundquist, F., Tygstrup, N., Winkler, K., Mellemgaard, K. & Munck-Petersen, S. Ethanol metabolism and production of free acetate in the human liver. J. Clin. Invest. 41, 955–961 (1962).
Seitz, H. K. & Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 7, 599–612 (2007).
Kendrick, S. F. et al. Acetate, the key modulator of inflammatory responses in acute alcoholic hepatitis. Hepatology 51, 1988–1997 (2010).
Schug, Z. T. et al. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell 27, 57–71 (2015). This study identified ACSS2 as a key enzyme for cancer cell growth under 'tumour-like' conditions of low lipid availability and hypoxia. It also showed that ACSS2 was highly upregulated under these same conditions and that depletion of ACSS2 inhibited tumour growth.
Comerford, S. A. et al. Acetate dependence of tumors. Cell 159, 1591–1602 (2014). This was the first paper to study Acss2−/− mice in two different mouse models of liver cancer and showed that loss of ACSS2 decreased tumour burden.
Fleming, S. E., Choi, S. Y. & Fitch, M. D. Absorption of short-chain fatty acids from the rat cecum in vivo. J. Nutr. 121, 1787–1797 (1991).
Reynolds, D. A., Rajendran, V. M. & Binder, H. J. Bicarbonate-stimulated [14C]butyrate uptake in basolateral membrane vesicles of rat distal colon. Gastroenterology 105, 725–732 (1993).
Chu, S. & Montrose, M. H. Extracellular pH regulation in microdomains of colonic crypts: effects of short-chain fatty acids. Proc. Natl Acad. Sci. USA 92, 3303–3307 (1995).
Miyauchi, S., Gopal, E., Fei, Y. J. & Ganapathy, V. Functional identification of SLC5A8, a tumor suppressor down-regulated in colon cancer, as a Na+-coupled transporter for short-chain fatty acids. J. Biol. Chem. 279, 13293–13296 (2004).
Coady, M. J. et al. The human tumour suppressor gene SLC5A8 expresses a Na+-monocarboxylate cotransporter. J. Physiol. 557, 719–731 (2004).
Li, H. et al. SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc. Natl Acad. Sci. USA 100, 8412–8417 (2003).
Lin, H. Y. et al. Protein expressions and genetic variations of SLC5A8 in prostate cancer risk and aggressiveness. Urology 78, 971.e1–971.e9 (2011).
Ullah, M. S., Davies, A. J. & Halestrap, A. P. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1α-dependent mechanism. J. Biol. Chem. 281, 9030–9037 (2006).
Parks, S. K., Chiche, J. & Pouyssegur, J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat. Rev. Cancer 13, 611–623 (2013).
Kirat, D. & Kato, S. Monocarboxylate transporter 1 (MCT1) mediates transport of short-chain fatty acids in bovine caecum. Exp. Physiol. 91, 835–844 (2006).
Pinheiro, C. et al. Increased expression of monocarboxylate transporters 1, 2, and 4 in colorectal carcinomas. Virchows Arch. 452, 139–146 (2008).
Moschen, I., Broer, A., Galic, S., Lang, F. & Broer, S. Significance of short chain fatty acid transport by members of the monocarboxylate transporter family (MCT). Neurochem. Res. 37, 2562–2568 (2012).
Jackson, V. N. & Halestrap, A. P. The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver cells determined using the fluorescent intracellular pH indicator, 2′,7′-bis(carboxyethyl)-5(6)-carboxyfluorescein. J. Biol. Chem. 271, 861–868 (1996).
Olivares, O., Dabritz, J. H., King, A., Gottlieb, E. & Halsey, C. Research into cancer metabolomics: towards a clinical metamorphosis. Semin. Cell Dev. Biol. 43, 52–64 (2015).
Bola, B. M. et al. Inhibition of monocarboxylate transporter-1 (MCT1) by AZD3965 enhances radiosensitivity by reducing lactate transport. Mol. Cancer Ther. 13, 2805–2816 (2014).
Fujino, T., Kondo, J., Ishikawa, M., Morikawa, K. & Yamamoto, T. T. Acetyl-CoA synthetase 2, a mitochondrial matrix enzyme involved in the oxidation of acetate. J. Biol. Chem. 276, 11420–11426 (2001).
Luong, A., Hannah, V. C., Brown, M. S. & Goldstein, J. L. Molecular characterization of human acetyl-CoA synthetase, an enzyme regulated by sterol regulatory element-binding proteins. J. Biol. Chem. 275, 26458–26466 (2000). This is a seminal paper describing the molecular characterization of transcriptional regulation of ACSS2.
Watkins, P. A., Maiguel, D., Jia, Z. & Pevsner, J. Evidence for 26 distinct acyl-coenzyme A synthetase genes in the human genome. J. Lipid Res. 48, 2736–2750 (2007).
Tucek, S. Subcellular distribution of acetyl-CoA synthetase, ATP citrate lyase, citrate synthase, choline acetyltransferase, fumarate hydratase and lactate dehydrogenase in mammalian brain tissue. J. Neurochem. 14, 531–545 (1967).
Hatzivassiliou, G. et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell 8, 311–321 (2005).
Zaidi, N., Royaux, I., Swinnen, J. V. & Smans, K. ATP citrate lyase knockdown induces growth arrest and apoptosis through different cell- and environment-dependent mechanisms. Mol. Cancer Ther. 11, 1925–1935 (2012).
Wellen, K. E. et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324, 1076–1080 (2009).
Bauer, D. E., Hatzivassiliou, G., Zhao, F., Andreadis, C. & Thompson, C. B. ATP citrate lyase is an important component of cell growth and transformation. Oncogene 24, 6314–6322 (2005).
Kim, J. W., Tchernyshyov, I., Semenza, G. L. & Dang, C. V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3, 177–185 (2006).
Spriet, L. L. et al. Pyruvate dehydrogenase activation and kinase expression in human skeletal muscle during fasting. J. Appl. Physiol. 96, 2082–2087 (2004).
Kamphorst, J. J., Chung, M. K., Fan, J. & Rabinowitz, J. D. Quantitative analysis of acetyl-CoA production in hypoxic cancer cells reveals substantial contribution from acetate. Cancer Metab. 2, 23 (2014). This paper provides the first description of the lipogenic substrate gap that occurs particularly under hypoxic conditions. It identified acetate as the additional source of lipogenic acetyl-CoA.
Mashimo, T. et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 159, 1603–1614 (2014). This is a landmark paper describing the tissue-specific enhancement of acetate oxidation by glioblastomas and brain metastases (regardless of their tissue of origin).
Kuhajda, F. P. et al. Fatty acid synthesis: a potential selective target for antineoplastic therapy. Proc. Natl Acad. Sci. USA 91, 6379–6383 (1994).
Xu, M. et al. An acetate switch regulates stress erythropoiesis. Nat. Med. 20, 1018–1026 (2014).
Maher, E. A. et al. Metabolism of [U-13C]glucose in human brain tumors in vivo. NMR Biomed. 25, 1234–1244 (2012). This was the original paper describing how the infusion of patients with stable isotope tracers revealed the bioenergetic substrate gap in brain tumours.
Bjornson, E. et al. Stratification of hepatocellular carcinoma patients based on acetate utilization. Cell Rep. 13, 2014–2026 (2015).
Schug, Z. T., Vande Voorde, J. & Gottlieb, E. The nurture of tumors can drive their metabolic phenotype. Cell Metab. 23, 391–392 (2016).
Davidson, S. M. et al. Environment impacts the metabolic dependencies of Ras-driven non-small cell lung cancer. Cell Metab. 23, 517–528 (2016).
McBrian, M. A. et al. Histone acetylation regulates intracellular pH. Mol. Cell 49, 310–321 (2013).
Takahashi, H., McCaffery, J. M., Irizarry, R. A. & Boeke, J. D. Nucleocytosolic acetyl-coenzyme a synthetase is required for histone acetylation and global transcription. Mol. Cell 23, 207–217 (2006). The role of acetyl-CoA synthetases in the regulation of histone acetylation was first identified in yeast.
Gao, X. et al. Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nat. Commun. 7, 11960 (2016).
Chen, R. et al. The acetate/ACSS2 switch regulates HIF-2 stress signaling in the tumor cell microenvironment. PLoS ONE 10, e0116515 (2015).
Fan, J., Krautkramer, K. A., Feldman, J. L. & Denu, J. M. Metabolic regulation of histone post-translational modifications. ACS Chem. Biol. 10, 95–108 (2015).
Lee, J. V. et al. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab. 20, 306–319 (2014).
Rajendran, P., Williams, D. E., Ho, E. & Dashwood, R. H. Metabolism as a key to histone deacetylase inhibition. Crit. Rev. Biochem. Mol. Biol. 46, 181–199 (2011).
Martinez-Pastor, B., Cosentino, C. & Mostoslavsky, R. A tale of metabolites: the cross-talk between chromatin and energy metabolism. Cancer Discov. 3, 497–501 (2013).
Yoshii, Y. et al. Cytosolic acetyl-CoA synthetase affected tumor cell survival under hypoxia: the possible function in tumor acetyl-CoA/acetate metabolism. Cancer Sci. 100, 821–827 (2009).
Sonveaux, P. et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Invest. 118, 3930–3942 (2008).
Houtkooper, R. H., Pirinen, E. & Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 13, 225–238 (2012).
Chen, R., Dioum, E. M., Hogg, R. T., Gerard, R. D. & Garcia, J. A. Hypoxia increases sirtuin 1 expression in a hypoxia-inducible factor-dependent manner. J. Biol. Chem. 286, 13869–13878 (2011).
Starai, V. J., Celic, I., Cole, R. N., Boeke, J. D. & Escalante-Semerena, J. C. Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science 298, 2390–2392 (2002).
Schwer, B., Bunkenborg, J., Verdin, R. O., Andersen, J. S. & Verdin, E. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc. Natl Acad. Sci. USA 103, 10224–10229 (2006).
Hallows, W. C., Lee, S. & Denu, J. M. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc. Natl Acad. Sci. USA 103, 10230–10235 (2006). References 72 and 73 were the first reports of the regulation of ACSS1 and ACSS2 by reversible acetylation, in part controlled by SIRTs.
Brown, A. J. et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 278, 11312–11319 (2003). This is a seminal paper that initially identified and characterized the acetate receptors.
Priyadarshini, M. et al. An acetate-specific GPCR, FFAR2, regulates insulin secretion. Mol. Endocrinol. 29, 1055–1066 (2015).
Tang, C. et al. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat. Med. 21, 173–177 (2015).
Ge, H. et al. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 149, 4519–4526 (2008).
Hong, Y. H. et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 146, 5092–5099 (2005).
Peck, B. et al. Inhibition of fatty acid desaturation is detrimental to cancer cell survival in metabolically compromised environments. Cancer Metab. 4, 6 (2016).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013).
Erny, D. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977 (2015).
Correa-Oliveira, R., Fachi, J. L., Vieira, A., Sato, F. T. & Vinolo, M. A. Regulation of immune cell function by short-chain fatty acids. Clin. Transl Immunol. 5, e73 (2016).
Hatanaka, H. et al. Identification of transforming activity of free fatty acid receptor 2 by retroviral expression screening. Cancer Sci. 101, 54–59 (2010).
Tang, Y., Chen, Y., Jiang, H., Robbins, G. T. & Nie, D. G-Protein-coupled receptor for short-chain fatty acids suppresses colon cancer. Int. J. Cancer 128, 847–856 (2011).
Yonezawa, T., Kobayashi, Y. & Obara, Y. Short-chain fatty acids induce acute phosphorylation of the p38 mitogen-activated protein kinase/heat shock protein 27 pathway via GPR43 in the MCF-7 human breast cancer cell line. Cell. Signal. 19, 185–193 (2007).
Shou, Y., Lu, J., Chen, T., Ma, D. & Tong, L. Correlation of fluorodeoxyglucose uptake and tumor-proliferating antigen Ki-67 in lymphomas. J. Cancer Res. Ther. 8, 96–102 (2012).
Riedl, C. C. et al. 18F-FDG PET scanning correlates with tissue markers of poor prognosis and predicts mortality for patients after liver resection for colorectal metastases. J. Nucl. Med. 48, 771–775 (2007).
Gillies, R. J., Schornack, P. A., Secomb, T. W. & Raghunand, N. Causes and effects of heterogeneous perfusion in tumors. Neoplasia 1, 197–207 (1999).
Yamamoto, Y. et al. 11C-acetate PET in the evaluation of brain glioma: comparison with 11C-methionine and 18F-FDG-PET. Mol. Imaging Biol. 10, 281–287 (2008).
Wong, T. Z., van der Westhuizen, G. J. & Coleman, R. E. Positron emission tomography imaging of brain tumors. Neuroimaging Clin. N. Am. 12, 615–626 (2002).
Yoshii, Y., Furukawa, T., Saga, T. & Fujibayashi, Y. Acetate/acetyl-CoA metabolism associated with cancer fatty acid synthesis: overview and application. Cancer Lett. 356, 211–216 (2015).
Vavere, A. L., Kridel, S. J., Wheeler, F. B. & Lewis, J. S. 1-11C-acetate as a PET radiopharmaceutical for imaging fatty acid synthase expression in prostate cancer. J. Nucl. Med. 49, 327–334 (2008).
Oyama, N. et al. 11C-acetate PET imaging of prostate cancer. J. Nucl. Med. 43, 181–186 (2002).
Ponde, D. E. et al. 18F-fluoroacetate: a potential acetate analog for prostate tumor imaging—in vivo evaluation of 18F-fluoroacetate versus 11C-acetate. J. Nucl. Med. 48, 420–428 (2007).
Jadvar, H. Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. J. Nucl. Med. 52, 81–89 (2011).
Brogsitter, C., Zophel, K. & Kotzerke, J. 18F-Choline, 11C-choline and 11C-acetate PET/CT: comparative analysis for imaging prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 40 (Suppl. 1), S18–S27 (2013).
Schiepers, C. et al. 1-11C-acetate kinetics of prostate cancer. J. Nucl. Med. 49, 206–215 (2008).
Mena, E. et al. 11C-Acetate PET/CT in localized prostate cancer: a study with MRI and histopathologic correlation. J. Nucl. Med. 53, 538–545 (2012).
Oyama, N. et al. 11C-Acetate PET imaging for renal cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 36, 422–427 (2009).
Park, J. W. et al. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J. Nucl. Med. 49, 1912–1921 (2008).
Lewis, D. Y. et al. Late imaging with [1-11C]acetate improves detection of tumor fatty acid synthesis with PET. J. Nucl. Med. 55, 1144–1149 (2014).
Tsuchida, T., Takeuchi, H., Okazawa, H., Tsujikawa, T. & Fujibayashi, Y. Grading of brain glioma with 1-11C-acetate PET: comparison with 18F-FDG PET. Nucl. Med. Biol. 35, 171–176 (2008).
Ho, C. L. et al. 11C-acetate PET/CT for metabolic characterization of multiple myeloma: a comparative study with 18F-FDG PET/CT. J. Nucl. Med. 55, 749–752 (2014).
Seltzer, M. A. et al. Radiation dose estimates in humans for 11C-acetate whole-body PET. J. Nucl. Med. 45, 1233–1236 (2004).
Hensley, C. T. et al. Metabolic heterogeneity in human lung tumors. Cell 164, 681–694 (2016).
Peters, S. G., Pomare, E. W. & Fisher, C. A. Portal and peripheral blood short chain fatty acid concentrations after caecal lactulose instillation at surgery. Gut 33, 1249–1252 (1992).
Dankert, J., Zijlstra, J. B. & Wolthers, B. G. Volatile fatty acids in human peripheral and portal blood: quantitative determination vacuum distillation and gas chromatography. Clin. Chim. Acta 110, 301–307 (1981).
Gregory, J. F. 3rd et al. Metabolomic analysis reveals extended metabolic consequences of marginal vitamin B-6 deficiency in healthy human subjects. PLoS ONE 8, e63544 (2013).
Pouteau, E. et al. Production rate of acetate during colonic fermentation of lactulose: a stable-isotope study in humans. Am. J. Clin. Nutr. 68, 1276–1283 (1998).
Pouteau, E., Meirim, I., Metairon, S. & Fay, L. B. Acetate, propionate and butyrate in plasma: determination of the concentration and isotopic enrichment by gas chromatography/mass spectrometry with positive chemical ionization. J. Mass Spectrom. 36, 798–805 (2001).
Robertson, M. D., Bickerton, A. S., Dennis, A. L., Vidal, H. & Frayn, K. N. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am. J. Clin. Nutr. 82, 559–567 (2005).
Pomare, E. W., Branch, W. J. & Cummings, J. H. Carbohydrate fermentation in the human colon and its relation to acetate concentrations in venous blood. J. Clin. Invest. 75, 1448–1454 (1985).
Akanji, A. O., Humphreys, S., Thursfield, V. & Hockaday, T. D. The relationship of plasma acetate with glucose and other blood intermediary metabolites in non-diabetic and diabetic subjects. Clin. Chim. Acta 185, 25–34 (1989).
Akanji, A. O., Ng, L. & Humphreys, S. Plasma acetate levels in response to intravenous fat or glucose/insulin infusions in diabetic and non-diabetic subjects. Clin. Chim. Acta 178, 85–94 (1988).
Crouse, J. R., Gerson, C. D., DeCarli, L. M. & Lieber, C. S. Role of acetate in the reduction of plasma free fatty acids produced by ethanol in man. J. Lipid Res. 9, 509–512 (1968).
Fernandes, J., Vogt, J. & Wolever, T. M. Inulin increases short-term markers for colonic fermentation similarly in healthy and hyperinsulinaemic humans. Eur. J. Clin. Nutr. 65, 1279–1286 (2011).
Simoneau, C. et al. Measurement of whole body acetate turnover in healthy subjects with stable isotopes. Biol. Mass Spectrom. 23, 430–433 (1994).
Tollinger, C. D., Vreman, H. J. & Weiner, M. W. Measurement of acetate in human blood by gas chromatography: effects of sample preparation, feeding, and various diseases. Clin. Chem. 25, 1787–1790 (1979).
Pownall, H. J. et al. Effect of moderate alcohol consumption on hypertriglyceridemia: a study in the fasting state. Arch. Intern. Med. 159, 981–987 (1999).
Muir, J. G. et al. Resistant starch in the diet increases breath hydrogen and serum acetate in human subjects. Am. J. Clin. Nutr. 61, 792–799 (1995).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
MetaboMed Ltd, Israel, Shareholder and Director (E.G.). Z.T.S. and J.V.V. declare no competing interests.
Glossary
- Crabtree effect
-
The phenomenon whereby aerobic fermentation of glucose occurs and respiration is inhibited if the glucose concentration surpasses a threshold value.
- Short-chain fatty acids
-
(SCFAs). Fatty acids with an aliphatic tail of two to six carbon atoms. The SCFAs acetate, propionate and butyrate are abundantly produced by bacterial fermentation of dietary carbohydrates in the colon.
- Facultative autotrophs
-
Organisms that can grow using organic carbon but are also able to assimilate organic carbon from inorganic carbon sources such as CO2.
- Lipogenic substrate gap
-
Alternative carbon sources that contribute to de novo fatty acid synthesis, as a large proportion (20–40%) of carbons in fatty acids in hypoxic cancer cells cannot be attributed to glucose or glutamine.
- Bioenergetic substrate gap
-
Alternative sources of carbon that contribute to tumour bioenergetics, as less than 50% of acetyl-CoA in human brain tumours is derived from blood-borne glucose.
- Crotonylation
-
A post-translational modification of histone lysine residues that has been shown to stimulate transcription and is, at least partially, dependent on acetyl-CoA synthetase 2 (ACSS2)-catalysed production of crotonyl-CoA from the short-chain fatty acid crotonate.
- Caloric restriction
-
Caloric restriction without malnutrition is a dietary strategy that has been studied extensively as a way to slow ageing and extend lifespan.
Rights and permissions
About this article
Cite this article
Schug, Z., Vande Voorde, J. & Gottlieb, E. The metabolic fate of acetate in cancer. Nat Rev Cancer 16, 708–717 (2016). https://doi.org/10.1038/nrc.2016.87
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc.2016.87
This article is cited by
-
Acetyl-CoA synthetase 2 contributes to a better prognosis for liver cancer by switching acetate-glucose metabolism
Experimental & Molecular Medicine (2024)
-
Butyrate’s (a short-chain fatty acid) microbial synthesis, absorption, and preventive roles against colorectal and lung cancer
Archives of Microbiology (2024)
-
Association between human blood metabolome and the risk of breast cancer
Breast Cancer Research (2023)
-
ACSS2-dependent histone acetylation improves cognition in mouse model of Alzheimer’s disease
Molecular Neurodegeneration (2023)
-
Short-chain fatty acids in diseases
Cell Communication and Signaling (2023)