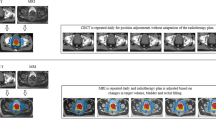
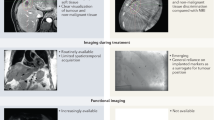
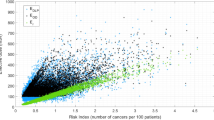
Key Points
-
Radiotherapy has proven potential to cure tumours by eradicating cancer stem cells.
-
Current state-of-the-art techniques of photon-based radiotherapy are approaching the physical limits of shaping high doses to the target volume.
-
Particle therapy reduces the volume of normal tissues irradiated with low or intermediate radiation doses and has the potential to reduce side effects in normal tissue as well as to escalate doses to radioresistant tumours.
-
Photon and especially particle radiotherapy will benefit from further improvements to image guidance with full adaptation of dose delivery to motion and anatomical changes of tumours and normal tissues during treatment.
-
Treatment planning algorithms that enable much faster planning and replanning, include uncertainties to improve robustness and enable optimization of multi-objective planning or plan comparison are on the verge of widespread clinical implementation.
-
The basic biological mechanisms of radiosensitivity and radioresistance are known and part of today's population-based treatment strategies.
-
Biomarkers and bio-imaging that enable mechanisms of radioresistance to be assessed in individual tumours and normal tissues are rapidly emerging.
-
Biomarkers have unexplored potential for predicting the extent of subclinical spread of tumour cells to help define the clinical target volume.
-
Biomarkers, when integrated into treatment planning, may potentiate the degree of personalization of radiotherapy that is already achieved today on a technological basis.
-
Radiation oncology has high potential to showcase the efficacy of precision medicine in oncology.
Abstract
Technological advances and clinical research over the past few decades have given radiation oncologists the capability to personalize treatments for accurate delivery of radiation dose based on clinical parameters and anatomical information. Eradication of gross and microscopic tumours with preservation of health-related quality of life can be achieved in many patients. Two major strategies, acting synergistically, will enable further widening of the therapeutic window of radiation oncology in the era of precision medicine: technology-driven improvement of treatment conformity, including advanced image guidance and particle therapy, and novel biological concepts for personalized treatment, including biomarker-guided prescription, combined treatment modalities and adaptation of treatment during its course.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bernier, J., Hall, E. J. & Giaccia, A. Radiation oncology: a century of achievements. Nat. Rev. Cancer 4, 737–747 (2004).
Verellen, D. et al. Innovations in image-guided radiotherapy. Nat. Rev. Cancer 7, 949–960 (2007).
Baumann, M., Krause, M. & Hill, R. Exploring the role of cancer stem cells in radioresistance. Nat. Rev. Cancer 8, 545–554 (2008).
Weichselbaum, R. R. & Hellman, S. Oligometastases revisited. Nat. Rev. Clin. Oncol. 8, 378–382 (2011).
Holthusen, H. Erfahrungen über die Verträglichkeitsgrenze für Röntgenstrahlen und deren Nutzanwendung zur Verhütung von Schäden. Strahlentherapie 57, 254–269 (1936).
Atun, R. et al. Expanding global access to radiotherapy. Lancet Oncol. 16, 1153–1186 (2015).
Minniti, G. et al. Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother. Oncol. 97, 377–381 (2010).
Auperin, A. et al. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 1764 patients. Ann. Oncol. 17, 473–483 (2006).
Baumann, M. et al. Final results of the randomized phase III CHARTWEL-trial (ARO 97–91) comparing hyperfractionated-accelerated versus conventionally fractionated radiotherapy in non-small cell lung cancer (NSCLC). Radiother. Oncol. 100, 76–85 (2011).
Torok, J. A. et al. Low-dose consolidation radiation therapy for early stage unfavorable Hodgkin lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 92, 54–59 (2015).
Eichenauer, D. A. et al. Long-term course of patients with stage IA nodular lymphocyte-predominant Hodgkin lymphoma: a report from the German Hodgkin Study Group. J. Clin. Oncol. 33, 2857–2862 (2015).
Nkhali, L. et al. FDG-PET/CT during concomitant chemo radiotherapy for esophageal cancer: reducing target volumes to deliver higher radiotherapy doses. Acta Oncol. 54, 909–915 (2015).
Zips, D. & Baumann, M. Place of proton radiotherapy in future radiotherapy practice. Semin. Radiat. Oncol. 23, 149–153 (2013).
Veldeman, L. et al. Evidence behind use of intensity-modulated radiotherapy: a systematic review of comparative clinical studies. Lancet Oncol. 9, 367–375 (2008).
Mackie, T. R. et al. Tomotherapy: a new concept for the delivery of dynamic conformal radiotherapy. Med. Phys. 20, 1709–1719 (1993).
Yu, C. X. Intensity-modulated arc therapy with dynamic multileaf collimation: an alternative to tomotherapy. Phys. Med. Biol. 40, 1435–1449 (1995).
Otto, K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med. Phys. 35, 310–317 (2008). The first viable approach to single-arc VMAT. Rapidly deployed in many clinics as a more time-efficient form of IMRT.
Cheng, C. W. et al. Commissioning and clinical implementation of a sliding gantry CT scanner installed in an existing treatment room and early clinical experience for precise tumor localization. Am. J. Clin. Oncol. 26, e28–e36 (2003).
Grusell, E. et al. Patient positioning for fractionated precision radiation treatment of targets in the head using fiducial markers. Radiother. Oncol. 33, 68–72 (1994).
Zhang, H. & Sonke, J. J. Directional interpolation for motion weighted 4D cone-beam CT reconstruction. Med. Image Comput. Comput. Assist. Interv. 15, 181–188 (2012).
Olsen, J., Green, O. & Kashani, R. World's first application of MR-guidance for radiotherapy. Mol. Med. 112, 358–360 (2015).
Lagendijk, J. J. et al. MRI/linac integration. Radiother. Oncol. 86, 25–29 (2008).
Tanner, R. L., Baily, N. A. & Hilbert, J. W. High-energy proton depth-dose patterns. Radiat. Res. 32, 861–874 (1967).
Jakel, O. Medical physics aspects of particle therapy. Radiat. Prot. Dosim. 137, 156–166 (2009).
Rong, Y. & Welsh, J. Basics of particle therapy II biologic and dosimetric aspects of clinical hadron therapy. Am. J. Clin. Oncol. 33, 646–649 (2010).
Palm, A. & Johansson, K. A. A review of the impact of photon and proton external beam radiotherapy treatment modalities on the dose distribution in field and out-of-field; implications for the long-term morbidity of cancer survivors. Acta Oncol. 46, 462–473 (2007).
Tubiana, M. Can we reduce the incidence of second primary malignancies occurring after radiotherapy? A critical review. Radiother. Oncol. 91, 4–15; discussion 1–3 (2009).
Paganetti, H. et al. Assessment of radiation-induced second cancer risks in proton therapy and IMRT for organs inside the primary radiation field. Phys. Med. Biol. 57, 6047–6061 (2012).
McGowan, S. E., Burnet, N. G. & Lomax, A. J. Treatment planning optimisation in proton therapy. Br. J. Radiol. 86, 20120288 (2013).
Mathieu, J. et al. HIF induces human embryonic stem cell markers in cancer cells. Cancer Res. 71, 4640–4652 (2011).
Kuess, P. et al. Automated evaluation of setup errors in carbon ion therapy using PET: feasibility study. Med. Phys. 40, 121718 (2013).
Enghardt, W., Fromm, W. D., Manfrass, P. & Schardt, D. Limited-angle 3D reconstruction of PET images for dose localization in light ion tumour therapy. Phys. Med. Biol. 37, 791–798 (1992).
Golnik, C. et al. Range assessment in particle therapy based on prompt gamma-ray timing measurements. Phys. Med. Biol. 59, 5399–5422 (2014).
Speers, C. et al. Development and validation of a novel radiosensitivity signature in human breast cancer. Clin. Cancer Res. 21, 3667–3677 (2015).
Liu, Q. et al. Lung cancer cell line screen links fanconi anemia/BRCA pathway defects to increased relative biological effectiveness of proton radiation. Int. J. Radiat. Oncol. Biol. Phys. 91, 1081–1089 (2015).
Karger, C. P., Peschke, P., Scholz, M., Huber, P. E. & Debus, J. Relative biological effectiveness of carbon ions in a rat prostate carcinoma in vivo: comparison of 1, 2, and 6 fractions. Int. J. Radiat. Oncol. Biol. Phys. 86, 450–455 (2013).
Peschke, P., Karger, C. P., Scholz, M., Debus, J. & Huber, P. E. Relative biological effectiveness of carbon ions for local tumor control of a radioresistant prostate carcinoma in the rat. Int. J. Radiat. Oncol. Biol. Phys. 79, 239–246 (2011).
Saager, M. et al. Carbon ion irradiation of the rat spinal cord: dependence of the relative biological effectiveness on linear energy transfer. Int. J. Radiat. Oncol. Biol. Phys. 90, 63–70 (2014).
Paganetti, H. et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int. J. Radiat. Oncol. Biol. Phys. 53, 407–421 (2002).
Fachal, L. et al. A three-stage genome-wide association study identifies a susceptibility locus for late radiotherapy toxicity at 2q24.1. Nat. Genet. 46, 891–894 (2014).
Wouters, B. G. et al. Radiobiological intercomparison of the 160 MeV and 230 MeV proton therapy beams at the Harvard Cyclotron Laboratory and at Massachusetts General Hospital. Radiat. Res. 183, 174–187 (2015).
Jones, B., Wilson, P., Nagano, A., Fenwick, J. & McKenna, G. Dilemmas concerning dose distribution and the influence of relative biological effect in proton beam therapy of medulloblastoma. Br. J. Radiol. 85, e912–e918 (2012).
Chaudhary, P. et al. Relative biological effectiveness variation along monoenergetic and modulated Bragg peaks of a 62-MeV therapeutic proton beam: a preclinical assessment. Int. J. Radiat. Oncol. Biol. Phys. 90, 27–35 (2014).
Asaithamby, A., Hu, B. & Chen, D. J. Unrepaired clustered DNA lesions induce chromosome breakage in human cells. Proc. Natl Acad. Sci. USA 108, 8293–8298 (2011).
Barendsen, G. W. RBE-LET relationships for different types of lethal radiation damage in mammalian cells: comparison with DNA dsb and an interpretation of differences in radiosensitivity. Int. J. Radiat. Biol. 66, 433–436 (1994).
Williams, M. V., Denekamp, J. & Fowler, J. F. A review of alpha/beta ratios for experimental tumors: implications for clinical studies of altered fractionation. Int. J. Radiat. Oncol. Biol. Phys. 11, 87–96 (1985).
Ma, N. Y., Tinganelli, W., Maier, A., Durante, M. & Kraft-Weyrather, W. Influence of chronic hypoxia and radiation quality on cell survival. J. Radiat. Res. 54 (Suppl. 1), i13–i22 (2013).
De Ruysscher, D. et al. Charged particles in radiotherapy: a 5-year update of a systematic review. Radiother. Oncol. 103, 5–7 (2012).
Lodge, M. et al. A systematic literature review of the clinical and cost-effectiveness of hadron therapy in cancer. Radiother. Oncol. 83, 110–122 (2007).
Haie-Meder, C., Siebert, F. A. & Potter, R. Image guided, adaptive, accelerated, high dose brachytherapy as model for advanced small volume radiotherapy. Radiother. Oncol. 100, 333–343 (2011).
Louie, A. V. et al. Inter-observer and intra-observer reliability for lung cancer target volume delineation in the 4D-CT era. Radiother. Oncol. 95, 166–171 (2010).
Jensen, N. K. et al. Dynamic contrast enhanced CT aiding gross tumor volume delineation of liver tumors: an interobserver variability study. Radiother. Oncol. 111, 153–157 (2014).
Ashamalla, H. et al. The contribution of integrated PET/CT to the evolving definition of treatment volumes in radiation treatment planning in lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 63, 1016–1023 (2005).
De Ruysscher, D., Nestle, U., Jeraj, R. & Macmanus, M. PET scans in radiotherapy planning of lung cancer. Lung Cancer 75, 141–145 (2012).
Moghaddasi, L., Bezak, E. & Marcu, L. G. Current challenges in clinical target volume definition: tumour margins and microscopic extensions. Acta Oncol. 51, 984–995 (2012).
Early Breast Cancer Trialists' Collaborative Group et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378, 1707–1716 (2011).
Yang, J. C. et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J. Clin. Oncol. 16, 197–203 (1998).
Salguero, F. J. et al. Microscopic disease extensions as a risk factor for loco-regional recurrence of NSCLC after SBRT. Radiother. Oncol. 109, 26–31 (2013).
Veronesi, U. et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol. 14, 1269–1277 (2013).
Vaidya, J. S. et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT—a randomised trial. Lancet 383, 603–613 (2014).
Witte, M. G. et al. Relating dose outside the prostate with freedom from failure in the Dutch trial 68 Gy versus 78 Gy. Int. J. Radiat. Oncol. Biol. Phys. 77, 131–138 (2010).
Heemsbergen, W. D., Al-Mamgani, A., Witte, M. G., van Herk, M. & Lebesque, J. V. Radiotherapy with rectangular fields is associated with fewer clinical failures than conformal fields in the high-risk prostate cancer subgroup: results from a randomized trial. Radiother. Oncol. 107, 134–139 (2013).
Engels, B. et al. Impact of planning target volume margins and rectal distention on biochemical failure in image-guided radiotherapy of prostate cancer. Radiother. Oncol. 111, 106–109 (2014).
Stroom, J. et al. Combined recipe for clinical target volume and planning target volume margins. Int. J. Radiat. Oncol. Biol. Phys. 88, 708–714 (2014).
Chen, W., Gilhuijs, K., Stroom, J., Bartelink, H. & Sonke, J. J. A simulation framework for modeling tumor control probability in breast conserving therapy. Radiother. Oncol. 111, 289–295 (2014).
Wanet, M. et al. Gradient-based delineation of the primary GTV on FDG-PET in non-small cell lung cancer: a comparison with threshold-based approaches, CT and surgical specimens. Radiother. Oncol. 98, 117–125 (2011).
Unkelbach, J. et al. Radiotherapy planning for glioblastoma based on a tumor growth model: implications for spatial dose redistribution. Phys. Med. Biol. 59, 771–789 (2014).
Durante, M. & Loeffler, J. S. Charged particles in radiation oncology. Nat. Rev. Clin. Oncol. 7, 37–43 (2010).
Seregni, M. et al. Tumor tracking based on correlation models in scanned ion beam therapy: an experimental study. Phys. Med. Biol. 58, 4659–4678 (2013).
Schatti, A., Zakova, M., Meer, D. & Lomax, A. J. The effectiveness of combined gating and re-scanning for treating mobile targets with proton spot scanning. An experimental and simulation-based investigation. Phys. Med. Biol. 59, 3813–3828 (2014).
Matney, J. et al. Effects of respiratory motion on passively scattered proton therapy versus intensity modulated photon therapy for stage III lung cancer: are proton plans more sensitive to breathing motion? Int. J. Radiat. Oncol. Biol. Phys. 87, 576–582 (2013).
Barnett, G. C. et al. A genome wide association study (GWAS) providing evidence of an association between common genetic variants and late radiotherapy toxicity. Radiother. Oncol. 111, 178–185 (2014).
Peguret, N. et al. Apnea-like suppression of respiratory motion: first evaluation in radiotherapy. Radiother. Oncol. in the press (2016).
Engels, B., Soete, G., Verellen, D. & Storme, G. Conformal arc radiotherapy for prostate cancer: increased biochemical failure in patients with distended rectum on the planning computed tomogram despite image guidance by implanted markers. Int. J. Radiat. Oncol. Biol. Phys. 74, 388–391 (2009).
Unkelbach, J., Chan, T. C. & Bortfeld, T. Accounting for range uncertainties in the optimization of intensity modulated proton therapy. Phys. Med. Biol. 52, 2755–2773 (2007). The first approach to dealing with range uncertainties in particle therapy through robust optimization.
Pflugfelder, D., Wilkens, J. J. & Oelfke, U. Worst case optimization: a method to account for uncertainties in the optimization of intensity modulated proton therapy. Phys. Med. Biol. 53, 1689–1700 (2008).
Fredriksson, A., Forsgren, A. & Hardemark, B. Minimax optimization for handling range and setup uncertainties in proton therapy. Med. Phys. 38, 1672–1684 (2011).
Yan, D., Vicini, F., Wong, J. & Martinez, A. Adaptive radiation therapy. Phys. Med. Biol. 42, 123–132 (1997).
Yan, D. Adaptive radiotherapy: merging principle into clinical practice. Semin. Radiat. Oncol. 20, 79–83 (2010).
Moller, D. S., Khalil, A. A., Knap, M. M. & Hoffmann, L. Adaptive radiotherapy of lung cancer patients with pleural effusion or atelectasis. Radiother. Oncol. 110, 517–522 (2014).
Chen, A. M. et al. Clinical outcomes among patients with head and neck cancer treated by intensity-modulated radiotherapy with and without adaptive replanning. Head Neck 36, 1541–1546 (2014).
Schwartz, D. L. et al. Adaptive radiotherapy for head and neck cancer—dosimetric results from a prospective clinical trial. Radiother. Oncol. 106, 80–84 (2013).
Langen, K. M. in Uncertainties in External Beam Radiation Therapy (eds Palta, J. R. & Mackie, T. R.) 443–470 (Medical Physics Publishing, 2011).
Sonke, J. J. & Belderbos, J. Adaptive radiotherapy for lung cancer. Semin. Radiat. Oncol. 20, 94–106 (2010).
Sovik, A., Malinen, E. & Olsen, D. R. Adapting biological feedback in radiotherapy. Semin. Radiat. Oncol. 20, 138–146 (2010).
Wu, B. et al. Patient geometry-driven information retrieval for IMRT treatment plan quality control. Med. Phys. 36, 5497–5505 (2009). The first analysis of how the spatial and geometric relationship between tumour target and crucial structures affects the dosimetric trade-off between the two. The concept is evolving into knowledge-based automated planning.
Moore, K. L., Brame, R. S., Low, D. A. & Mutic, S. Experience-based quality control of clinical intensity-modulated radiotherapy planning. Int. J. Radiat. Oncol. Biol. Phys. 81, 545–551 (2011).
Lin, A. & Maity, A. Molecular pathways: a novel approach to targeting hypoxia and improving radiotherapy efficacy via reduction in oxygen demand. Clin. Cancer Res. 21, 1995–2000 (2015).
Raleigh, J. A. et al. Hypoxia and vascular endothelial growth factor expression in human squamous cell carcinomas using pimonidazole as a hypoxia marker. Cancer Res. 58, 3765–3768 (1998).
Servagi-Vernat, S. et al. Hypoxia-guided adaptive radiation dose escalation in head and neck carcinoma: a planning study. Acta Oncol. 54, 1008–1016 (2015).
Tramm, T. et al. Development and validation of a gene profile predicting benefit of postmastectomy radiotherapy in patients with high-risk breast cancer: a study of gene expression in the DBCG82bc cohort. Clin. Cancer Res. 20, 5272–5280 (2014).
Bokrantz, R. & Forsgren, A. An algorithm for approximating convex pareto surfaces based on dual techniques. INFORMS J. Comput. 25, 377–393 (2013).
Jee, K. W., McShan, D. L. & Fraass, B. A. Lexicographic ordering: intuitive multicriteria optimization for IMRT. Phys. Med. Biol. 52, 1845–1861 (2007).
Wilkens, J. J., Alaly, J. R., Zakarian, K., Thorstad, W. L. & Deasy, J. O. IMRT treatment planning based on prioritizing prescription goals. Phys. Med. Biol. 52, 1675–1692 (2007).
Monz, M., Kufer, K. H., Bortfeld, T. R. & Thieke, C. Pareto navigation: algorithmic foundation of interactive multi-criteria IMRT planning. Phys. Med. Biol. 53, 985–998 (2008).
Craft, D. L., Hong, T. S., Shih, H. A. & Bortfeld, T. R. Improved planning time and plan quality through multicriteria optimization for intensity-modulated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 82, e83–e90 (2012). The first demonstration of the clinical benefit of multi-criteria optimization (MCO), in terms of both improved plan quality and reduced planning time.
Wala, J., Craft, D., Paly, J., Zietman, A. & Efstathiou, J. Maximizing dosimetric benefits of IMRT in the treatment of localized prostate cancer through multicriteria optimization planning. Med. Dosim. 38, 298–303 (2013).
Petersen, C. et al. Repopulation of FaDu human squamous cell carcinoma during fractionated radiotherapy correlates with reoxygenation. Int. J. Radiat. Oncol. Biol. Phys. 51, 483–493 (2001).
Yaromina, A. et al. Radiobiological hypoxia, histological parameters of tumour microenvironment and local tumour control after fractionated irradiation. Radiother. Oncol. 96, 116–122 (2010).
Yaromina, A. et al. Exploratory study of the prognostic value of microenvironmental parameters during fractionated irradiation in human squamous cell carcinoma xenografts. Int. J. Radiat. Oncol. Biol. Phys. 80, 1205–1213 (2011).
Baumann, M., Dubois, W. & Suit, H. D. Response of human squamous cell carcinoma xenografts of different sizes to irradiation: relationship of clonogenic cells, cellular radiation sensitivity in vivo, and tumor rescuing units. Radiat. Res. 123, 325–330 (1990).
Hill, R. P. & Milas, L. The proportion of stem cells in murine tumors. Int. J. Radiat. Oncol. Biol. Phys. 16, 513–518 (1989).
Krause, M., Yaromina, A., Eicheler, W., Koch, U. & Baumann, M. Cancer stem cells: targets and potential biomarkers for radiotherapy. Clin. Cancer Res. 17, 7224–7229 (2011).
Withers, H. R. in Advances in Radiation Biology (eds Lett, J. T. & Adler, H.) 241–247 (Academic Press, 1975).
Dorr, W. Three A's of repopulation during fractionated irradiation of squamous epithelia: asymmetry loss, acceleration of stem-cell divisions and abortive divisions. Int. J. Radiat. Biol. 72, 635–643 (1997).
Dorr, W. Radiobiological models of normal tissue reactions. Strahlenther. Onkol. 174 (Suppl. 3), 4–7 (1998).
Bentzen, S. M., Harari, P. M. & Bernier, J. Exploitable mechanisms for combining drugs with radiation: concepts, achievements and future directions. Nat. Clin. Pract. Oncol. 4, 172–180 (2007).
Krause, M. et al. Decreased repopulation as well as increased reoxygenation contribute to the improvement in local control after targeting of the EGFR by C225 during fractionated irradiation. Radiother. Oncol. 76, 162–167 (2005).
Overgaard, J. et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet 362, 933–940 (2003).
Overgaard, J. et al. A randomized double-blind phase III study of nimorazole a a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5–85. Radiother. Oncol. 46, 135–146 (1998). Example of a clinical trial demonstrating improved local tumour control after radiotherapy by tackling, in a population-based approach, radiobiological mechanisms of radioresistance (in this tumour, hypoxia by the hypoxic cell sensitizer nimorazole).
Bentzen, J. et al. Locally advanced head and neck cancer treated with accelerated radiotherapy, the hypoxic modifier nimorazole and weekly cisplatin. Results from the DAHANCA 18 phase II study. Acta Oncol. 54, 1001–1007 (2015).
Overgaard, J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck—a systematic review and meta-analysis. Radiother. Oncol. 100, 22–32 (2011).
Corry, J. & Rischin, D. Strategies to overcome accelerated repopulation and hypoxia—what have we learned from clinical trials? Semin. Oncol. 31, 802–808 (2004).
Mauguen, A. et al. Hyperfractionated or accelerated radiotherapy in lung cancer: an individual patient data meta-analysis. J. Clin. Oncol. 30, 2788–2797 (2012).
Pignon, J. P., le Maitre, A., Maillard, E. & Bourhis, J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother. Oncol. 92, 4–14 (2009).
Bonner, J. A. et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 354, 567–578 (2006).
Bonner, J. A. et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 11, 21–28 (2010).
Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J. Clin. Oncol. 26, 5802–5812 (2008).
Wopken, K. et al. Development of a multivariable normal tissue complication probability (NTCP) model for tube feeding dependence after curative radiotherapy/chemo-radiotherapy in head and neck cancer. Radiother. Oncol. 113, 95–101 (2014).
Beetz, I. et al. External validation of three dimensional conformal radiotherapy based NTCP models for patient-rated xerostomia and sticky saliva among patients treated with intensity modulated radiotherapy. Radiother. Oncol. 105, 94–100 (2012).
Beetz, I. et al. Development of NTCP models for head and neck cancer patients treated with three-dimensional conformal radiotherapy for xerostomia and sticky saliva: the role of dosimetric and clinical factors. Radiother. Oncol. 105, 86–93 (2012).
Bentzen, S. M. & Overgaard, J. Patient-to-patient variability in the expression of radiation-induced normal tissue injury. Semin. Radiat. Oncol. 4, 68–80 (1994).
Kozin, S. V. et al. Inter- and intramouse heterogeneity of radiation response for a growing paired organ. Radiat. Res. 170, 264–267 (2008).
Krause, M., Gurtner, K., Deuse, Y. & Baumann, M. Heterogeneity of tumour response to combined radiotherapy and EGFR inhibitors: differences between antibodies and TK inhibitors. Int. J. Radiat. Biol. 85, 943–954 (2009).
Schutze, C. et al. Effect of increase of radiation dose on local control relates to pre-treatment FDG uptake in FaDu tumours in nude mice. Radiother. Oncol. 83, 311–315 (2007).
Schutze, C. et al. Effect of [18F]FMISO stratified dose-escalation on local control in FaDu hSCC in nude mice. Radiother. Oncol. 111, 81–87 (2014).
Collins, G. S., Reitsma, J. B., Altman, D. G. & Moons, K. G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Br. J. Cancer 112, 251–259 (2015).
Lohaus, F. et al. HPV16 DNA status is a strong prognosticator of loco-regional control after postoperative radiochemotherapy of locally advanced oropharyngeal carcinoma: results from a multicentre explorative study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Radiother. Oncol. 113, 317–323 (2014).
Linge, A. et al. Low cancer stem cell marker expression and low hypoxia identify good prognosis subgroups in HPV− HNSCC after postoperative radiochemotherapy: a multicenter study of the DKTK-ROG. Clin. Cancer Res. http://dx.doi.org/10.1158/1078-0432.CCR-15-1990 (2016). Clinical study on the prognostic value of several biomarkers, including cancer stem cell density and tumour hypoxia, for outcome of postoperative radiochemotherapy of head and neck cancers, indicating that biomarkers may provide a basis for biologically driven treatment modification in individual patients.
Lohaus, F. et al. Einfluss von Tumorvolumen und p16 Status auf die lokoregionäre Kontrolle von Kopf-Hals-Tumoren nach primärer Radiochemotherapie: Ergebnisse einer multizentrischen Biomarker-Studie des Deutschen Konsortiums für Translationale Krebsforschung (DKTK-ROG). Abstract V-08-06. Strahlenther. Onkol. 191 (Suppl.), S31 (2015).
West, C. M., Dunning, A. M. & Rosenstein, B. S. Genome-wide association studies and prediction of normal tissue toxicity. Semin. Radiat. Oncol. 22, 91–99 (2012).
West, C. M. & Barnett, G. C. Genetics and genomics of radiotherapy toxicity: towards prediction. Genome Med. 3, 52 (2011).
Rosenstein, B. S. et al. Radiogenomics: Radiobiology enters the era of big data and team science. Int. J. Radi. Oncol. Biol. Phys. 89, 709–713 (2014).
Zips, D. et al. Exploratory prospective trial of hypoxia-specific PET imaging during radiochemotherapy in patients with locally advanced head-and-neck cancer. Radiother. Oncol. 105, 21–28 (2012).
Zschaeck, S. et al. Spatial distribution of FMISO in head and neck squamous cell carcinomas during radio-chemotherapy and its correlation to pattern of failure. Acta Oncol. 54, 1355–1363 (2015).
Hentschel, M. et al. Early FDG PET at 10 or 20 Gy under chemoradiotherapy is prognostic for locoregional control and overall survival in patients with head and neck cancer. Eur. J. Nucl. Med. Mol. Imag. 38, 1203–1211 (2011).
Eicheler, W., Krause, M., Hessel, F., Zips, D. & Baumann, M. Kinetics of EGFR expression during fractionated irradiation varies between different human squamous cell carcinoma lines in nude mice. Radiother. Oncol. 76, 151–156 (2005).
Zips, D. et al. Prognostic value of radiobiological hypoxia during fractionated irradiation for local tumor control. Strahlenther. Onkol. 187, 306–310 (2011).
Cojoc, M. et al. Aldehyde dehydrogenase is regulated by β-catenin/TCF and promotes radioresistance in prostate cancer progenitor cells. Cancer Res. 75, 1482–1494 (2015).
Kurth, I. et al. Cancer stem cell related markers of radioresistance in head and neck squamous cell carcinoma. Oncotarget 6, 34494–34509 (2015).
Peitzsch, C. et al. Targeting of the epigenetic reprogramming for prostate cancer cell radiosensitization. Cancer Res. in the press (2016).
Fisher, E. R. et al. Pathologic findings from the National Surgical Adjuvant Breast Project (protocol no. 4). I. Observations concerning the multicentricity of mammary cancer. Cancer 35, 247–254 (1975).
Coleman, C. N., Lawrence, T. S. & Kirsch, D. G. Enhancing the efficacy of radiation therapy: premises, promises, and practicality. J. Clin. Oncol. 32, 2832–2835 (2014).
Krause, M., Zips, D., Thames, H. D., Kummermehr, J. & Baumann, M. Preclinical evaluation of molecular-targeted anticancer agents for radiotherapy. Radiother. Oncol. 80, 112–122 (2006).
Ling, C. C. et al. Perspectives of multidimensional conformal radiation treatment. Radiother. Oncol. 29, 129–139 (1993).
Ling, C. C. et al. Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int. J. Radiat. Oncol. Biol. Phys. 47, 551–560 (2000). The first conceptual description of the idea of dose painting, taking tumour heterogeneity into account.
Eschwege, F. et al. Predictive assays of radiation response in patients with head and neck squamous cell carcinoma: a review of the Institute Gustave Roussy experience. Int. J. Radiat. Oncol. Biol. Phys. 39, 849–853 (1997).
Menegakis, A. et al. Residual γH2AX foci after ex vivo irradiation of patient samples with known tumour-type specific differences in radio-responsiveness. Radiother. Oncol. 116, 480–485 (2015).
Menegakis, A. et al. γH2AX assay in ex vivo irradiated tumour specimens: a novel method to determine tumour radiation sensitivity in patient-derived material. Radiother. Oncol. 116, 473–479 (2015).
Ang, K. K. et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 363, 24–35 (2010). A clinical study of the prognostic value of HPV status for outcome of primary radiotherapy of HNSCC, indicating that HPV biomarker may provide a basis for biologically stratified treatment modification in individual patients.
Cellini, F., Morganti, A. G., Genovesi, D., Silvestris, N. & Valentini, V. Role of microRNA in response to ionizing radiations: evidences and potential impact on clinical practice for radiotherapy. Molecules 19, 5379–5401 (2014).
Toustrup, K. et al. Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer Res. 71, 5923–5931 (2011).
Eustace, A. et al. A 26-gene hypoxia signature predicts benefit from hypoxia-modifying therapy in laryngeal cancer but not bladder cancer. Clin. Cancer Res. 19, 4879–4888 (2013).
Pajonk, F. & Vlashi, E. Characterization of the stem cell niche and its importance in radiobiological response. Semin. Radiat. Oncol. 23, 237–241 (2013).
Eriksen, J. G. et al. Molecular profiles as predictive marker for the effect of overall treatment time of radiotherapy in supraglottic larynx squamous cell carcinomas. Radiother. Oncol. 72, 275–282 (2004).
Eriksen, J. G., Steiniche, T., Askaa, J., Alsner, J. & Overgaard, J. The prognostic value of epidermal growth factor receptor is related to tumor differentiation and the overall treatment time of radiotherapy in squamous cell carcinomas of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 58, 561–566 (2004).
Eriksen, J. G., Alsner, J., Steiniche, T. & Overgaard, J. The possible role of TP53 mutation status in the treatment of squamous cell carcinomas of the head and neck (HNSCC) with radiotherapy with different overall treatment times. Radiother. Oncol. 76, 135–142 (2005).
Eriksen, J. G., Steiniche, T. & Overgaard, J. The influence of epidermal growth factor receptor and tumor differentiation on the response to accelerated radiotherapy of squamous cell carcinomas of the head and neck in the randomized DAHANCA 6 and 7 study. Radiother. Oncol. 74, 93–100 (2005).
De Jong, M. C. et al. CD44 expression predicts local recurrence after radiotherapy in larynx cancer. Clin. Cancer Res. 16, 5329–5338 (2010).
Butof, R., Dubrovska, A. & Baumann, M. Clinical perspectives of cancer stem cell research in radiation oncology. Radiother. Oncol. 108, 388–396 (2013).
Aerts, H. J. et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 5, 4006 (2014).
Schwitalla, S. et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell 152, 25–38 (2013).
Nagano, O., Okazaki, S. & Saya, H. Redox regulation in stem-like cancer cells by CD44 variant isoforms. Oncogene 32, 5191–5198 (2013).
Harris, I. S. et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 27, 211–222 (2015).
Jakobi, A. et al. Identification of patient benefit from proton therapy for advanced head and neck cancer patients based on individual and subgroup normal tissue complication probability analysis. Int. J. Radiat. Oncol. Biol. Phys. 92, 1165–1174 (2015).
Toustrup, K. et al. Gene expression classifier predicts for hypoxic modification of radiotherapy with nimorazole in squamous cell carcinomas of the head and neck. Radiother. Oncol. 102, 122–129 (2012).
Roelofs, E. et al. International data-sharing for radiotherapy research: an open-source based infrastructure for multicentric clinical data mining. Radiother. Oncol. 110, 370–374 (2014).
Skripcak, T. et al. Creating a data exchange strategy for radiotherapy research: Towards federated databases and anonymised public datasets. Radiother. Oncol. 113, 303–309 (2014).
Lagadec, C. et al. Tumor cells with low proteasome subunit expression predict overall survival in head and neck cancer patients. BMC Cancer 14, 152 (2014).
De Ruysscher, D. et al. First report on the patient database for the identification of the genetic pathways involved in patients over-reacting to radiotherapy: GENEPI–II. Radiother. Oncol. 97, 36–39 (2010).
De Ruysscher, D. et al. Quantification of radiation-induced lung damage with CT scans: the possible benefit for radiogenomics. Acta Oncol. 52, 1405–1410 (2013).
Pajonk, F. & McBride, W. H. The proteasome in cancer biology and treatment. Radiat. Res. 156, 447–459 (2001).
Chang, C. W. et al. Distinct subpopulations of head and neck cancer cells with different levels of intracellular reactive oxygen species exhibit diverse stemness, proliferation, and chemosensitivity. Cancer Res. 74, 6291–6305 (2014).
Lopez-Chavez, A. et al. Molecular profiling and targeted therapy for advanced thoracic malignancies: a biomarker-derived, multiarm, multihistology phase II basket trial. J. Clin. Oncol. 33, 1000–1007 (2015).
Redig, A. J. & Janne, P. A. Basket trials and the evolution of clinical trial design in an era of genomic medicine. J. Clin. Oncol. 33, 975–977 (2015).
Langendijk, J. A. et al. Selection of patients for radiotherapy with protons aiming at reduction of side effects: the model-based approach. Radiother. Oncol. 107, 267–273 (2013). Description of a model-based approach to selecting patients for a new treatment using response models for tumours and normal tissues. In a second phase the outcome of the patients is compared with a matched historical cohort or in some situations by randomized trials to validate the models.
Relton, C., Torgerson, D., O'Cathain, A. & Nicholl, J. Rethinking pragmatic randomised controlled trials: introducing the “cohort multiple randomised controlled trial” design. Br. Med. J. 340, c1066 (2010).
Rischin, D. et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J. Clin. Oncol. 28, 4142–4148 (2010).
Habbous, S. et al. Comorbidity and prognosis in head and neck cancers: Differences by subsite, stage, and human papillomavirus status. Head Neck 36, 802–810 (2014).
Brahme, A. & Agren, A. K. Optimal dose distribution for eradication of heterogeneous tumours. Acta Oncol. 26, 377–385 (1987).
Tome, W. A. & Fowler, J. F. Selective boosting of tumor subvolumes. Int. J. Radiat. Oncol. Biol. Phys. 48, 593–599 (2000).
Bentzen, S. M. Theragnostic imaging for radiation oncology: dose-painting by numbers. Lancet Oncol. 6, 112–117 (2005).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT01341535 (2015).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT01429493 (2015).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT01411332 (2016).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT01212354 (2016).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02125175 (2016).
Berwouts, D. et al. Three-phase adaptive dose-painting-by-numbers for head-and-neck cancer: initial results of the phase I clinical trial. Radiother. Oncol. 107, 310–316 (2013).
Olteanu, L. A. et al. Comparative dosimetry of three-phase adaptive and non-adaptive dose-painting IMRT for head-and-neck cancer. Radiother. Oncol. 111, 348–353 (2014).
Vogelius, I. R. et al. Failure-probability driven dose painting. Med. Phys. 40, 081717 (2013).
Brahme, A. Biologically optimized 3-dimensional in vivo predictive assay-based radiation therapy using positron emission tomography-computerized tomography imaging. Acta Oncol. 42, 123–136 (2003).
Lyhne, N. M. et al. The DAHANCA 6 randomized trial: effect of 6 versus 5 weekly fractions of radiotherapy in patients with glottic squamous cell carcinoma. Radiother. Oncol. 117, 91–98 (2015).
Eriksen, J. et al. DAHANCA 19: First results of a randomized phase III study of the importance of the EGFR-inhibitor zalutumumab for the outcome of primary curative radiotherapy for squamous cell carcinoma of the head and neck. Eur. J. Cancer 49 (Suppl. 3), S6 abstract LBA12 (2013).
Overgaard, J. et al. Randomized study of darbepoetin alfa as modifier of radiotherapy in patients with primary squamous cell carcinoma of the head and neck (HNSCC): final outcome of the DAHANCA 10 trial. J. Clin. Oncol. 27 (Suppl.), abstract 6007 (2009).
Pignon, J. P., le Maitre, A. & Bourhis, J. Meta-analyses of chemotherapy in head and neck cancer (MACH-NC): an update. Int. J. Radiat. Oncol. Biol. Phys. 69, S112–S114 (2007).
Soliman, M. et al. GTV differentially impacts locoregional control of non-small cell lung cancer (NSCLC) after different fractionation schedules: subgroup analysis of the prospective randomized CHARTWEL trial. Radiother. Oncol. 106, 299–304 (2013).
Ball, D. L. et al. The complex relationship between lung tumor volume and survival in patients with non-small cell lung cancer treated by definitive radiotherapy: a prospective, observational prognostic factor study of the Trans-Tasman Radiation Oncology Group (TROG 99.05). Radiother. Oncol. 106, 305–311 (2013).
Basaki, K. et al. Prognostic factors for survival in stage III non-small-cell lung cancer treated with definitive radiation therapy: impact of tumor volume. Int. J. Radiat. Oncol. Biol. Phys. 64, 449–454 (2006).
Bradley, J. D. et al. Gross tumor volume, critical prognostic factor in patients treated with three-dimensional conformal radiation therapy for non-small-cell lung carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 52, 49–57 (2002).
Dehing-Oberije, C. et al. Tumor volume combined with number of positive lymph node stations is a more important prognostic factor than TNM stage for survival of non-small-cell lung cancer patients treated with (chemo)radiotherapy. Int. J. Radi. Oncol. Biol. Phys. 70, 1039–1044 (2008).
Dubben, H. H., Thames, H. D. & Beck-Bornholdt, H. P. Tumor volume: a basic and specific response predictor in radiotherapy [see comments]. Radiother. Oncol. 47, 167–174 (1998).
Etiz, D. et al. Influence of tumor volume on survival in patients irradiated for non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 53, 835–846 (2002).
Johnson, C. R., Thames, H. D., Huang, D. T. & Schmidt-Ullrich, R. K. The tumor volume and clonogen number relationship: tumor control predictions based upon tumor volume estimates derived from computed tomography. Int. J. Radiat. Oncol. Biol. Phys. 33, 281–287 (1995).
Baumann, M., Dubois, W., Pu, A., Freeman, J. & Suit, H. D. Response of xenografts of human malignant gliomas and squamous cell carcinomas to fractionated irradiation. Int. J. Radi. Oncol. Biol. Phys. 23, 803–810 (1992).
Suit, H., Skates, S., Taghian, A., Okunieff, P. & Efird, J. T. Clinical implications of heterogeneity of tumor response to radiation therapy. Radiother. Oncol. 25, 251–260 (1992).
Yaromina, A. et al. Pre-treatment number of clonogenic cells and their radiosensitivity are major determinants of local tumour control after fractionated irradiation. Radiother. Oncol. 83, 304–310 (2007).
Koch, U. et al. Residual γH2AX foci predict local tumour control after radiotherapy. Radiother. Oncol. 108, 434–439 (2013).
Menegakis, A. et al. Residual DNA double strand breaks in perfused but not in unperfused areas determine different radiosensitivity of tumours. Radiother. Oncol. 100, 137–144 (2011).
Menegakis, A. et al. Prediction of clonogenic cell survival curves based on the number of residual DNA double strand breaks measured by γH2AX staining. Int. J. Radiat. Biol. 85, 1032–1041 (2009).
Olive, P. L. & Banath, J. P. Phosphorylation of histone H2AX as a measure of radiosensitivity. Int. J. Radiat. Oncol. Biol. Phys. 58, 331–335 (2004).
Banath, J. P. & Olive, P. L. Expression of phosphorylated histone H2AX as a surrogate of cell killing by drugs that create DNA double-strand breaks. Cancer Res. 63, 4347–4350 (2003).
Chavaudra, N., Bourhis, J. & Foray, N. Quantified relationship between cellular radiosensitivity, DNA repair defects and chromatin relaxation: a study of 19 human tumour cell lines from different origin. Radiother. Oncol. 73, 373–382 (2004).
Koerber, S. A. et al. Influence of human papillomavirus and p16INK4a on treatment outcome of patients with anal cancer. Radiother. Oncol. 113, 331–336 (2014).
Lassen, P. et al. Impact of HPV-associated p16-expression on radiotherapy outcome in advanced oropharynx and non-oropharynx cancer. Radiother. Oncol. 113, 310–316 (2014).
Rieckmann, T. et al. HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother. Oncol. 107, 242–246 (2013).
Lassen, P. et al. The influence of HPV-associated p16-expression on accelerated fractionated radiotherapy in head and neck cancer: evaluation of the randomised DAHANCA 6&7 trial. Radiother. Oncol. 100, 49–55 (2011).
Lassen, P., Overgaard, J. & Eriksen, J. G. Expression of EGFR and HPV-associated p16 in oropharyngeal carcinoma: Correlation and influence on prognosis after radiotherapy in the randomized DAHANCA 5 and 7 trials. Radiother. Oncol. 108, 489–494 (2013).
Yaromina, A. et al. Pimonidazole labelling and response to fractionated irradiation of five human squamous cell carcinoma (hSCC) lines in nude mice: The need for a multivariate approach in biomarker studies. Radiother. Oncol. 81, 122–129 (2006).
Nordsmark, M., Overgaard, M. & Overgaard, J. Pretreatment oxygen predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother. Oncol. 41, 31–39 (1996).
Nordsmark, M. & Overgaard, J. A confirmatory prognostic study on oxygenation status and loco-regional control in advanced squamous cell carcinoma treated by radiation therapy. Radiother. Oncol. 57, 39–43 (2000). Important clinical dataset showing the impact of tumour hypoxia on local tumour control after radiotherapy in HNSCC.
Horsman, M. R., Mortensen, L. S., Petersen, J. B., Busk, M. & Overgaard, J. Imaging hypoxia to improve radiotherapy outcome. Nat. Rev. Clin. Oncol. 9, 674–687 (2012).
Kaanders, J. H. et al. Pimonidazole binding and tumor vascularity predict for treatment outcome in head and neck cancer. Cancer Res. 62, 7066–7074 (2002).
Nordsmark, M. et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother. Oncol. 77, 18–24 (2005).
Höckel, M. et al. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother. Oncol. 26, 45–50 (1993).
Movsas, B. et al. Hypoxic prostate/muscle pO2 ratio predicts for biochemical failure in patients with prostate cancer: preliminary findings. Urology 60, 634–639 (2002).
Rudat, V. et al. Predictive value of the tumor oxygenation by means of PO2 histography in patients with advanced head and neck cancer. Strahlenther. Onkol. 177, 462–468 (2001).
Kaanders, J. H. et al. ARCON: experience in 215 patients with advanced head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 52, 769–778 (2002).
Bentzen, S. M. et al. Epidermal growth factor receptor expression in pretreatment biopsies from head and neck squamous cell carcinoma as a predictive factor for a benefit from accelerated radiation therapy in a randomized controlled trial. J. Clin. Oncol. 23, 5560–5567 (2005).
Petersen, C. et al. Proliferation and micromilieu during fractionated irradiation of human FaDu squamous cell carcinoma in nude mice. Int. J. Radiat. Biol. 79, 469–477 (2003).
Butof, R. & Baumann, M. Time in radiation oncology — keep it short! Radiother. Oncol. 106, 271–275 (2013).
Baumann, M., Dorr, W., Petersen, C. & Krause, M. Repopulation during fractionated radiotherapy: much has been learned, even more is open. Int. J. Radiat. Biol. 79, 465–467 (2003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J.O. is listed as a co-inventor on a provisional patent application for a method of determining clinically relevant hypoxia in cancer that is owned by Aarhus University, Aarhus, Denmark, and the part concerning prediction of benefit from nimorazole is licensed to Azanta Denmark A/S, Hellerup, Denmark. All other authors declare no competing interests.
Related links
Glossary
- Cancer stem cells
-
(CSCs). Tumour cells that have unlimited potential to proliferate and to repopulate a whole tumour. For radiation oncology, CSCs are cells that have the potential to cause recurrences if they survive treatment.
- Radiation dose conformity
-
3D-planned radiotherapy with application of the prescribed high dose to the target volume and low dose to surrounding areas. Can be measured by the conformity index, which is the ratio between the planning target volume (PTV) and the irradiated volume. This ratio is ideally as high as possible, but cannot exceed a value of 1.
- Intensity-modulated radiation therapy
-
(IMRT). Characterized by inhomogeneous dose distribution within each individual radiation field. The resulting cumulative dose distribution usually achieves better target coverage with protection of normal tissues for irregular target volumes.
- Image-guided radiation therapy
-
(IGRT). Positioning control of organs and/or target volumes using in-room imaging modalities (X-ray, computed tomography (CT), magnetic resonance imaging (MRI)) directly, before application of the radiation fraction and correction of patient position accordingly.
- Multileaf collimators
-
(MLCs). Devices made from individually movable leaves that block the beam and can thereby form the treatment field.
- Stereotactic radiotherapy
-
Photon multi-field or arc radiotherapy that uses external coordinates for localization of the target. Usually applied to relatively small target volumes, for example, brain metastases or metastases in the body.
- Gantry
-
Part of the treatment machine that can be rotated around the patient and thereby apply the beam from different directions.
- Tomotherapy
-
Type of radiation therapy in which radiation is delivered slice by slice by a rotating beam.
- Volumetric modulated arc therapy
-
(VMAT). Image-guided radiation therapy technique with application of radiation during rotation of the gantry around the patient and continuous adaptation of the field formation (leaf positions) to the beam direction.
- Cone beam computed tomography
-
(CBCT). CT made from a rotating imaging source and detector, part of many modern radiotherapy machines.
- Fiducial markers
-
Small markers placed in the field of view of an imaging system, used in radiotherapy as reference for the matching of images, for example, for positioning control.
- MR-LINAC
-
Integrated linear accelerator with magnetic resonance imaging for image-guided radiotherapy.
- MR-cobalt machine
-
Integrated cobalt-60-based irradiation device with magnetic resonance imaging for image-guided radiotherapy.
- Particle therapy with protons
-
Radiotherapy with light ions (H+). Characterized by steep dose fall-off behind the target volume and relative biological effectiveness (RBE) values of 1.1–1.2 or, according to newer data, potentially up to 2 in specific areas of the beam.
- Reverse depth dose profile
-
Describes the depth dose profile of particles (protons or heavy ions) inside the body and is an increase in the radiation dose with penetration depth. This is inverse to photon radiotherapy, in which the dose decreases with depth.
- Bragg peak
-
A peak in the depth dose curve of particles (protons or heavy ions). The dose behind the Bragg peak is very small or zero. The depth of the Bragg peak depends on the energy of the beam. To cover a complete target volume in depth, the Bragg peak is spread out by combining several beam energies.
- Passive scattering
-
Spreading of the proton beam within the target volume is achieved by scattering material placed into the path of the beam; field borders are defined by absorber material.
- Active spot scanning
-
The beam scans the treatment volume voxel by voxel.
- Heavy ion beams
-
Heavier particles used for radiotherapy, most frequently carbon (12C). Characterized by steep dose fall-off behind the target volume and higher relative biological effectiveness (RBE) (∼2.3).
- Gross tumour volume
-
(GTV). The visible macroscopic tumour in the imaging modalities used for radiotherapy treatment planning.
- Clinical target volume
-
(CTV). Consists of the visible tumour (gross tumour volume, GTV) plus a volume of suspected microscopic spread.
- Planning target volume
-
(PTV). Consists of the clinical target volume (CTV) plus a margin for technical or positioning uncertainties, including movement of target volumes.
- Phantom studies
-
Studies of, for example, dose distribution using a phantom that relates to the essential human structures, densities or processes. For example, for dose distribution experiments, water-filled phantoms are used and for experiments on breathing-related motion, moving phantoms are used.
- Pareto optimality
-
A state in which it is not possible to improve one feature without worsening another.
- Intrinsic radiosensitivity
-
Radiosensitivity of an individual tumour independent of the impact of microenvironment or of fractionation parameters.
- Repopulation
-
Increase in the number of cancer stem cells between irradiation fractions by accelerated proliferation of surviving cells or reduced spontaneous cell loss between irradiation fractions.
- Prognostic assay
-
Gives information on the likely course of a disease.
- Predictive assay
-
In addition to the prognostic assay, gives information on the outcome of a specific treatment and thereby guides the optimal treatment for an individual patient.
- Phosphorylated histone H2AX
-
(γH2AX). Contributes to nucleosome formation and structure of DNA, and is phosphorylated as a consequence of DNA double-strand breaks.
- Single-arm model-based prospective trials
-
Non-randomized trials that select patients for specific treatments based on outcome models gained from previous treatments and, in the case of radiotherapy, based on radiation dose distributions for the actual patient.
- Interventional matrix trials
-
Clinical trials that build on patients with the same disease and treatment situation and use several biomarkers as a basis for treatment intervention. Positivity for one biomarker or biomarker combination may provide an intervention.
- Cohort multiple randomized controlled trials
-
A large cohort of patients with the disease of interest is recruited. For each trial, all eligible patients in the cohort are identified, and the group to receive the trial intervention is randomly selected. Outcomes of these patients are compared with those of eligible patients who were not randomly selected for the intervention.
- Rapid learning health care approaches
-
Computer-based data collection of treatment and outcomes of patients, development of prediction models based on the collected data, application of this knowledge to patients in clinical practice and comparison of predicted and actual outcomes to refine the models.
Rights and permissions
About this article
Cite this article
Baumann, M., Krause, M., Overgaard, J. et al. Radiation oncology in the era of precision medicine. Nat Rev Cancer 16, 234–249 (2016). https://doi.org/10.1038/nrc.2016.18
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc.2016.18
This article is cited by
-
Gene expression signature predicts radiation sensitivity in cell lines using the integral of dose–response curve
BMC Cancer (2024)
-
Prognostic biomarkers for the response to the radiosensitizer nimorazole combined with RCTx: a pre-clinical trial in HNSCC xenografts
Journal of Translational Medicine (2023)
-
Harnessing progress in radiotherapy for global cancer control
Nature Cancer (2023)
-
Predicting tumour radiosensitivity to deliver precision radiotherapy
Nature Reviews Clinical Oncology (2023)
-
Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions
Signal Transduction and Targeted Therapy (2023)