Abstract
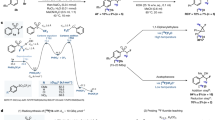
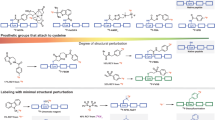
Proteins previously derivatized with the cross-coupling reagent sulfo-SMCC (4-(N-maleimidomethyl)cyclohexane-1-carboxylic acid 3-sulfo-N-hydroxy-succinimide ester sodium salt) can be easily labeled in high radiochemical yields with the silicon-fluoride acceptor (SiFA) reagent [18F]SiFA-SH, obtained via isotopic exchange, by thiol-maleimide coupling chemistry (n = 10). The specific activity of SiFA-SH obtained in a one-step labeling reaction was >18.5 GBq μmol−1 (>500 Ci mmol−1). The number of SiFA building blocks per protein molecule is defined by the previously introduced number of maleimide groups, which can be determined by a simple and convenient Ellman's assay. Not more than two maleimide groups are introduced using sulfo-SMCC, thereby keeping the modification of the protein low and preserving its biological activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
23 October 2012
In the version of this article initially published online, Katy Orchowski's name was spelled incorrectly. The surname Orchovski has been corrected to read Orchowski. The error has been corrected in all versions of this article.
References
Kostikov, A.P. et al. Synthesis of [18F]SiFB: a prosthetic group for direct protein radiolabeling for application in positron emission tomography. Nat. Protoc. 7, 1956–1963 (2012).
Wängler, C. et al. One-step 18F-labeling of peptides for positron emission tomography imaging using the SiFA methodology. Nat. Protoc. 7, 1946–1955 (2012).
Wängler, C. et al. Protein based imaging agents for in vivo positron-emission-tomography: a universally applicable gallium-68 labeling technique. J. Nucl. Med. 52, 586–591 (2011).
Ting, R., Adam, M.J., Ruth, T.J. & Perrin, D.M. Arylfluoroborates and alkylfluorosilicates as potential PET imaging agents: high-yielding aqueous biomolecular F-18-labeling. J. Am. Chem. Soc. 127, 13094–13095 (2005).
Li, Y. et al. Towards kit-like 18F-labeling of marimastat, a noncovalent inhibitor drug for in vivo PET imaging cancer associated matrix metalloproteases. Med. Chem. Commun. 2, 942–949 (2011).
Ting, R. et al. Toward [18F]-labeled aryltrifluoroborate radiotracers: in vivo positron emission tomography imaging of stable aryltrifluoroborate clearance in mice. J. Am. Chem. Soc. 130, 12045–12055 (2008).
McBride, W.J. et al. A novel method of 18F radiolabeling for PET. J. Nucl. Med. 50, 991–998 (2009).
McBride, W.J. et al. Improved 18F labeling of peptides with a fluoride-aluminum-chelate complex. Bioconjug. Chem. 21, 1331–1340 (2010).
McBride, W.J., D'Souza, C.A., Sharkey, R.M. & Goldenberg, D.M. The radiolabeling of proteins by the [18F]AlF method. Appl. Radiat. Isot. 70, 200–204 (2012).
Cai, L., Lu, S. & Pike, V.W. Chemistry with [18F]fluoride ion. Eur. J. Org. Chem. 2008, 2853–2873 (2008).
Smith, G.E., Sladen, H.L., Biagini, C.G. & Blower, P.J. Inorganic approaches for radiolabeling biomolecules with fluorine-18 for imaging with positron emission tomography. Dalton Trans. 40, 6196–6205 (2001).
Wu, Z. & Kandeel, F. 18F-labeled proteins. Curr. Pharm. Biotechnol. 11, 572–580 (2010).
Wester, H.J. & Schottelius, M. Fluorine-18 labeling of peptides and proteins. Ernst Schering Res. Found. Workshop 62, 79–111 (2007).
Vaidyanathan, G. & Zalutsky, M.R. Labeling proteins with fluorine-18 using N-succinimidyl 4-[18F]fluorobenzoate. Nucl. Med. Biol. 19, 275–281 (1992).
Brikh, A. & Morin, C. Boronated thiophenols: a preparation of 4-mercaptophenylboronic acid and derivatives. J. Organomet. Chem. 581, 82–86 (1999).
Acknowledgements
We thank the cyclotron crew of the McConnell Brain Imaging Centre for providing fluorine-18. The financial support of the following grant agencies are gratefully acknowledged: Canada Foundation for Innovation project no. 203639 to R.S., Natural Sciences and Engineering Research Council of Canada (NSERC) Strategic Project Grant Program (STPGP) no. 412893 to R.S., Bayern-Quebec-Allianz to S.N., Deutsche Forschungsgemeinschaft (DFG) grant no. WA 2132/3-1 to B.W. and Fonds der Chemischen Industrie to C.W.
Author information
Authors and Affiliations
Contributions
A.P.K., S.N., J.C., K.O., E.S., L.I.-B., K.J. and C.W. performed the experimental work. R.S., C.W. and B.W. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wängler, B., Kostikov, A., Niedermoser, S. et al. Protein labeling with the labeling precursor [18F]SiFA-SH for positron emission tomography. Nat Protoc 7, 1964–1969 (2012). https://doi.org/10.1038/nprot.2012.111
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2012.111
This article is cited by
-
Novel [18F]-labeled thiol for the labeling of Dha- or maleimide-containing biomolecules
EJNMMI Radiopharmacy and Chemistry (2022)
-
Protocols for clinical use at Nature Protocols
Nature Protocols (2021)
-
Direct fluorine-18 labeling of heat-sensitive biomolecules for positron emission tomography imaging using the Al18F-RESCA method
Nature Protocols (2018)
-
Characterization and optimization of heroin hapten-BSA conjugates: method development for the synthesis of reproducible hapten-based vaccines
Analytical and Bioanalytical Chemistry (2014)
-
One-step 18F-labeling of peptides for positron emission tomography imaging using the SiFA methodology
Nature Protocols (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.