Abstract
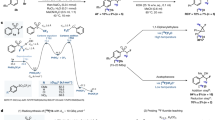
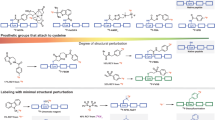
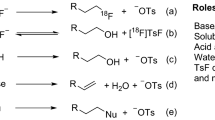
Here we present a procedure to label peptides with the positron-emitting radioisotope fluorine-18 (18F) using the silicon-fluoride acceptor (SiFA) labeling methodology. Positron emission tomography (PET) has gained high importance in noninvasive imaging of various diseases over the past decades, and thus new specific imaging probes for PET imaging, especially those labeled with 18F, because of the advantageous properties of this nuclide, are highly sought after. N-terminally SiFA–modified peptides can be labeled with 18F− in one step at room temperature (20–25 °C) or below without forming side products, thereby producing satisfactory radiochemical yields of 46 ± 1.5% (n = 10). The degree of chemoselectivity of the 18F-introduction, which is based on simple isotopic exchange, allows for a facile cartridge-based purification fully devoid of HPLC implementation, thereby yielding peptides with specific activities between 44.4 and 62.9 GBq μmol−1 (1,200–1,700 Ci mmol−1) within 25 min.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
23 October 2012
In the version of this article initially published online, Katy Orchowski's name was spelled incorrectly. The surname Orchovski has been corrected to read Orchowski. The error has been corrected in all versions of this article.
References
Schirrmacher, R. et al. F-18-labeling of peptides by means of an organosilicon-based fluoride acceptor. Angew. Chem. Int. Ed. Engl. 45, 6047–6050 (2006).
Wängler, C. et al. Silicon-[18F]fluorine radiochemistry: basics, applications and challenges. Appl. Sci. 2, 277–302 (2012).
Wängler, B. et al. Protein labeling with the labeling precursor [18F]SiFA-SH for positron emission tomography. Nat. Protoc. 7, 1964–1969 (2012).
Wängler, C. et al. One-step (18)F-labeling of carbohydrate-conjugated octreotate-derivatives containing a silicon-fluoride-acceptor (SiFA): in vitro and in vivo evaluation as tumor imaging agents for positron emission tomography (PET). Bioconjug. Chem. 21, 2289–2296 (2010).
Iovkova-Berends, L. et al. t-Bu(2)SiF-derivatized D(2)-receptor ligands: the first SiFA-containing small molecule radiotracers for target-specific PET-imaging. Molecules 16, 7458–7479 (2011).
Iovkova, L. et al. Para-functionalized aryl-di-tert-butylfluorosilanes as potential labeling synthons for F-18 radiopharmaceuticals. Chemistry 15, 2140–2147 (2009).
Kostikov, A.P. et al. N-(4-(Di-tert-butyl[(18)F]fluorosilyl)benzyl)-2-hydroxy-N,N-dimethylethylammonium bromide ([(18)F]SiFAN(+)Br(−)): a novel lead compound for the development of hydrophilic SiFA-based prosthetic groups for (18)F-labeling. J. Fluorine Chem. 132, 27–34 (2011).
Schirrmacher, E. et al. Synthesis of p-(di-tert-butyl[(18)f]fluorosilyl)benzaldehyde ([F-18]SiFA-A) with high specific activity by isotopic exchange: a convenient Labeling synthon for the F-18-labeling of n-amino-oxy derivatized peptides. Bioconjug. Chem. 18, 2085–2089 (2007).
Kostikov, A.P. et al. Synthesis of [18F]SiFB: a prosthetic group for direct protein radiolabeling for application in positron emission tomography. Nat. Protoc. 7, 1956–1963 (2012).
Mu, L.J. et al. Silicon-based building blocks for one-step (18)F-radiolabeling of peptides for PET imaging. Angew. Chem. Int. Engl. 47, 4922–4925 (2008).
Höhne, A. et al. Organofluorosilanes as model compounds for F-18-labeled silicon-based PET tracers and their hydrolytic stability: experimental data and theoretical calculations (PET = positron emission tomography). Chemistry 15, 3736–3743 (2009).
Höhne, A. et al. Synthesis, F-18-labeling, and in vitro and in vivo studies of bombesin peptides modified with silicon-based building blocks. Bioconjug. Chem. 19, 1871–1879 (2008).
Balentova, E. et al. Synthesis and hydrolytic stability of novel 3-[(18)F]fluoroethoxybis (1-methylethyl)silyl]propanamine-based prosthetic groups. J. Fluorine Chem. 132, 250–257 (2011).
Wessmann, S.H., Henriksen, G. & Wester, H.J. Cryptate mediated nucleophilic F-18-fluorination without azeotropic drying. Nuklearmedizin 51, 1–8 (2012).
Gill, H.S. & Marik, J. Preparation of F-18-labeled peptides using the copper(I)-catalyzed azide-alkyne 1,3-dipolar cycloaddition. Nat. Protoc. 6, 1718–1725 (2011).
Marik, J. & Sutcliffe, J.L. Click for PET: rapid preparation of [F-18]fluoropeptides using Cu-I catalyzed 1,3-dipolar cycloaddition. Tetrahedron Lett. 47, 6681–6684 (2006).
Glaser, M. & Arstad, E. 'Click labeling' with 2-[F-18]fluoroethylazide for positron emission tomography. Bioconjug. Chem. 18, 989–993 (2007).
Tietze, L.F. & Schmuck, K. SiFA azide: a new building block for PET imaging using click chemistry. Synlett 1697–1700 (2011).
Maschauer, S. et al. Labeling and glycosylation of peptides using click chemistry: a general approach to (18)F-glycopeptides as effective imaging probes for positron emission tomography. Angew. Chem. Int. Engl. 49, 976–979 (2010).
Ramenda, T., Kniess, T., Bergmann, R., Steinbach, J. & Wuest, F. Radiolabelling of proteins with fluorine-18 via click chemistry. Chem. Commun. 7521–7523 (2009).
Gill, H.S. et al. A modular platform for the rapid site-specific radiolabeling of proteins with F-18 exemplified by quantitative positron emission tomography of human epidermal growth factor receptor 2. J. Med. Chem. 52, 5816–5825 (2009).
Roehn, U. et al. Nucleophilic ring-opening of activated aziridines: a one-step method for labeling biomolecules with fluorine-18. J. Fluorine Chem. 130, 902–912 (2009).
Becaud, J. et al. Direct one-step(18)F-labeling of peptides via nucleophilic aromatic substitution. Bioconjug. Chem. 20, 2254–2261 (2009).
Jacobson, O. et al. Rapid and simple one-step F-18 labeling of peptides. Bioconjug. Chem. 22, 422–428 (2011).
Olberg, D.E. et al. One step radiosynthesis of 6-[F-18]fluoronicotinic acid 2,3,5,6-tetrafluorophenyl ester ([F-18]F-Py-TFP): a new prosthetic group for efficient labeling of biomolecules with fluorine-18. J. Med. Chem. 53, 1732–1740 (2010).
Vaidyanathan, G. & Zalutsky, M.R. Improved synthesis of N-succinimidyl 4-[F-18]fluorobenzoate and its application to the labeling of a monoclonal-antibody fragment. Bioconjug. Chem. 5, 352–356 (1994).
Wester, H.J., Hamacher, K. & Stocklin, G. A comparative study of NCA fluorine-18 labeling of proteins via acylation and photochemical conjugation. Nucl. Med. Biol. 23, 365–372 (1996).
Wuest, F., Kohler, L., Berndt, M. & Pietzsch, J. Systematic comparison of two novel, thiol-reactive prosthetic groups for F-18 labeling of peptides and proteins with the acylation agent succinimidyl-4-[F-18]fluorobenzoate ([F-18]SFB). Amino Acids 36, 283–295 (2009).
Glaser, M. et al. Methods for F-18-labeling of RGD peptides: comparison of aminooxy [F-18]fluorobenzaldehyde condensation with 'click labeling' using 2-[F-18]fluoroethylazide, and S-alkylation with [F-18]fluoropropanethiol. Amino Acids 37, 717–724 (2009).
Berndt, M., Pietzsch, J. & Wuest, F. Labeling of low-density lipoproteins using the F-18-labeled thiol-reactive N-[6-(4-[F-18]fluorobenzylidene)aminooxyhexyl]maleimide. Nucl. Med. Biol. 34, 5–15 (2007).
Cai, W.B., Zhang, X.Z., Wu, Y. & Chen, X. A thiol-reactive F-18-labeling agent, N-[2-(4-F-18-fluorobenzamido)ethyl]maleimide, and synthesis of RGD peptide-based tracer for PET imaging of α(v)β(3) integrin expression. J. Nucl. Med. 47, 1172–1180 (2006).
Poethko, T. et al. Two-step methodology for high-yield routine radiohalogenation of peptides: F-18-labeled RGD and octreotide analogs. J. Nucl. Med. 45, 892–902 (2004).
Inkster, J.A.H., Guerin, B., Ruth, T.J. & Adam, M.J. Radiosynthesis and bioconjugation of [F-18]FPy5yne, a prosthetic group for the F-18 labeling of bioactive peptides. J. Labelled Compd. Radpharm. 51, 444–452 (2008).
Namavari, M. et al. A novel method for direct site-specific radiolabeling of peptides using [F-18]FDG. Bioconjug. Chem. 20, 432–436 (2009).
Bruus-Jensen, K. et al. Chemoselective hydrazone formation between HYNIC-functionalized peptides and F-18-fluorinated aldehydes. Nucl. Med. Biol. 33, 173–183 (2006).
Arumugam, S., Chin, J., Schirrmacher, R., Popik, V.V. & Kostikov, A.P. [F-18] Azadibenzocyclooctyne ([F-18]ADIBO): a biocompatible radioactive labeling synthon for peptides using catalyst free [3+2] cycloaddition. Bioorg. Med. Chem. Lett. 21, 6987–6991 (2011).
Sutcliffe-Goulden, J.L., O'Doherty, M.J. & Bansal, S.S. Solid phase synthesis of [F-18]labelled peptides for positron emission tomography. Bioorg. Med. Chem. Lett. 10, 1501–1503 (2000).
Sutcliffe-Goulden, J.L. et al. Rapid solid phase synthesis and biodistribution of F-18-labelled linear peptides. Eur. J. Nucl. Med. Mol. Imaging 29, 754–759 (2002).
Marik, J., Hausner, S.H., Fix, L.A., Gagnon, M.K.J. & Sutcliffe, J.L. Solid-phase synthesis of 2-[F-18]fluoropropionyl peptides. Bioconjug. Chem. 17, 1017–1021 (2006).
Cai, L.S., Lu, S.Y. & Pike, V.W. Chemistry with [F-18]fluoride ion. Eur. J. Org. Chem. 2008, 2853–2873 (2008).
Smith, G.E., Sladen, H.L., Biagini, S.C.G. & Blower, P.J. Inorganic approaches for radiolabelling biomolecules with fluorine-18 for imaging with positron emission tomography. Dalton Trans. 40, 6196–6205 (2011).
Mu, L., Schubiger, A.P. & Ametamey, S.M. [18F]Fluorosilicon- and [18F]fluoroboron-based biomolecules for PET imaging. Curr. Radiopharm. 3, 224–242 (2010).
Mamat, C., Ramenda, T. & Wuest, F.R. Recent applications of click chemistry for the synthesis of radiotracers for molecular imaging. Mini Rev. Org. Chem. 6, 21–34 (2009).
Wängler, C., Schirrmacher, R., Bartenstein, P. & Wängler, B. Click-chemistry reactions in radiopharmaceutical chemistry: fast and easy introduction of radiolabels into biomolecules for in vivo imaging. Curr. Med. Chem. 17, 1092–1116 (2010).
Glaser, M. & Robins, E.G. 'Click labelling' in PET radiochemistry. J. Labelled Compd. Radpharm. 52, 407–414 (2009).
Ting, R., Adam, M.J., Ruth, T.J. & Perrin, D.M. Arylfluoroborates and alkylfluorosilicates as potential PET imaging agents: High-yielding aqueous biomolecular F-18-labeling. J. Am. Chem. Soc. 127, 13094–13095 (2005).
Li, Y. et al. Towards kit-like F-18-labeling of marimastat, a noncovalent inhibitor drug for in vivo PET imaging cancer associated matrix metalloproteases. Medchemcomm 2, 942–949 (2011).
Ting, R. et al. Toward [(18)F]-labeled aryltrifluoroborate radiotracers: in vivo positron emission tomography imaging of stable aryltrifluoroborate clearance in mice. J. Am. Chem. Soc. 130, 12045–12055 (2008).
McBride, W.J. et al. A novel method of (18)F radiolabeling for PET. J. Nucl. Med. 50, 991–998 (2009).
McBride, W.J. et al. Improved (18)F labeling of peptides with a fluoride-aluminum-chelate complex. Bioconjug. Chem. 21, 1331–1340 (2010).
McBride, W.J., D'Souza, C.A., Karacay, H., Sharkey, R.M. & Goldenberg, D.M. New lyophilized kit for rapid radiofluorination of peptides. Bioconjug. Chem. 2012, 538 (2012).
D'Souza, C.A., McBride, W.J., Sharkey, R.M., Todaro, L.J. & Goldenberg, D.M. High-yielding aqueous (18)F-labeling of peptides via Al(18)F chelation. Bioconjug. Chem. 22, 1793–1803 (2011).
Hamacher, K., Coenen, H.H. & Stocklin, G. Efficient stereospecific synthesis of no-carrier-added 2-[F-18]-fluoro-2-deoxy-D-glucose using aminopolyether supported nucleophilic-substitution. J. Nucl. Med. 27, 235–238 (1986).
Wellings, D.A. & Atherton, E. Standard Fmoc protocols. Methods Enzymol. 289, 44–67 (1997).
Patgiri, A., Menzenski, M.Z., Mahon, A.B. & Arora, P.S. Solid-phase synthesis of short α-helices stabilized by the hydrogen bond surrogate approach. Nat. Protoc. 5, 1857–1865 (2010).
Acknowledgements
We thank the cyclotron crew of the McConnell Brain Imaging Centre for providing fluorine-18. The financial support of the following grant agencies is gratefully acknowledged: Canada Foundation for Innovation (CFI) project no. 203639 to R.S., Natural Sciences and Engineering Research Council of Canada (NSERC) Strategic Project Grant Program (STPGP) no. 412893 to R.S., Bayern-Quebec-Allianz to S.N., Deutsche Forschungsgemeinschaft (DFG) grant no. WA 2132/3-1 to B.W., and Fonds der Chemischen Industrie to C.W.
Author information
Authors and Affiliations
Contributions
A.P.K., S.N., J.C., K.O., E.S., L.I.-B., K.J. and C.W. performed the experimental work. C.W., B.W. and R.S. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Video 1
18F-labeling of peptides and proteins using the SiFA protocol, Part 1 (AVI 29017 kb)
Supplementary Video 2
18F-labeling of peptides and proteins using the SiFA protocol, Part 2 (AVI 32890 kb)
Rights and permissions
About this article
Cite this article
Wängler, C., Niedermoser, S., Chin, J. et al. One-step 18F-labeling of peptides for positron emission tomography imaging using the SiFA methodology. Nat Protoc 7, 1946–1955 (2012). https://doi.org/10.1038/nprot.2012.109
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2012.109
This article is cited by
-
Novel [18F]-labeled thiol for the labeling of Dha- or maleimide-containing biomolecules
EJNMMI Radiopharmacy and Chemistry (2022)
-
Automated synthesis of [18F]Ga-rhPSMA-7/ -7.3: results, quality control and experience from more than 200 routine productions
EJNMMI Radiopharmacy and Chemistry (2021)
-
Kit-based synthesis of 2-deoxy-2-[18F]-fluoro-d-sorbitol for bacterial imaging
Nature Protocols (2021)
-
Protocols for clinical use at Nature Protocols
Nature Protocols (2021)
-
Radiosynthesis of [18F]SiFAlin-TATE for clinical neuroendocrine tumor positron emission tomography
Nature Protocols (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.