Abstract
The primary objective of this project was to determine the α4β2* nicotinic acetylcholine receptor (nAChR) occupancy in human brain of a single low dose of varenicline (0.5 mg), and to explore the relationship between receptor occupancy by varenicline and tobacco withdrawal symptoms (*denoting other putative nAChR subunits). Otherwise healthy smokers (n=9) underwent two positron emission tomography (PET) sessions with the selective α4β2* radioligand 2-FA. For the PET sessions, participants received either a low dose of varenicline (0.5 mg) or matching placebo pill (double-blind, random order) before imaging. For both sessions, participants received bolus plus continuous infusions of 2-FA, were scanned for 1 h after allowing the radiotracer to reach a steady state, smoked to satiety, and were scanned for 2 more hours. We estimated the fractional receptor occupancy by a single dose of varenicline (0.5 mg) and the corresponding varenicline dissociation constant (KV), along with the effect of low-dose varenicline, pill placebo, and smoking-to-satiety on withdrawal rating scales. The data are compatible with 100% occupancy of α4β2* nAChRs by a single dose of varenicline, with a 90% lower confidence limit of 89% occupancy for the thalamus and brainstem. The corresponding 90% upper limit on effective KV with respect to plasma varenicline was 0.49 nM. Smoking to satiety, but not low-dose varenicline, significantly reduced withdrawal symptoms. Our findings demonstrate that low-dose varenicline results in saturation of α4β2* nAChRs in the thalamus and brainstem without reducing withdrawal symptoms.
Similar content being viewed by others
INTRODUCTION
Tobacco use is the leading cause of preventable death in the United States, with an estimated 100 billion dollars spent on healthcare costs, annually, associated with the condition. Prolonged tobacco use is a major risk factor for a number of illnesses, including cancer, cardiovascular disease, and emphysema. The consequences of tobacco use are far reaching, influencing others through second- (Brody et al, 2011) and third-hand (Ueta et al, 2010) exposure, as well as causing developmental exposure during pregnancy (Lotfipour et al, 2009, 2010; Toledo-Rodriguez et al, 2010). Given the many risks associated with tobacco use and the global consequences, it is surprising that over a billion people continue to smoke worldwide. For these reasons, researchers have aimed to understand the mechanisms mediating tobacco addiction for the purpose of developing prevention and intervention strategies to reduce the health costs associated with this pandemic condition.
Clinical and preclinical evidence suggest that nicotine represents a primary constituent in tobacco smoke leading to reward and withdrawal (Brody et al, 2006, 2009). Binding to nicotinic acetylcholine receptors (nAChRs) throughout the brain and body, nicotine mimics the properties of endogenous acetylcholine, which is known to influence reward, attention, and memory, with continued exposure leading to long-term neurochemical adaptations in the brain. This mechanism is proposed to underlie the withdrawal syndrome precipitated by smoking cessation (Changeux, 2010).
Tobacco withdrawal is a primary cause of relapse, thereby perpetuating use (Kenny and Markou, 2001). The withdrawal syndrome is characterized by both affective (psychological) and somatic (physical) components, including craving, irritability, trouble in sleeping, anxiety, body tremors, headaches, weight gain, and depression (Hughes, 2007a; Kenny and Markou, 2001). Symptoms of withdrawal peak early after smoking cessation (Hatsukami et al, 1991; Hughes, 2007a), and have been shown to be alleviated through smoking-cessation therapies that act on nAChRs and can persist for many weeks (Hatsukami et al, 1991; Hughes, 2007a). To alleviate withdrawal, thereby reducing relapse in smokers, researchers have focused their attention on smoking-cessation therapies that affect nAChRs (Fowler et al, 2008; West et al, 2008).
Nicotinic receptors are pentameric ligand-gated ion channels, comprised of either homomeric alpha (α7, 9) or heteromeric alpha (α2-7, 9, 10) and beta (β2-4) subunits (Changeux, 2010). The α4β2* nAChR is the most common and widely distributed nicotinic receptor assembly in the brain (*denoting other putative nAChR subunits) (Lotfipour et al, 2011b). A large body of evidence, however, exists for the role of non-α4β2* nicotinic receptor subunits in mediating nicotine withdrawal (eg, α2, α5, or β4), with results suggesting that certain nAChR subunits, but not others, differentially regulate somatic and affective nicotine-withdrawal symptoms (Changeux, 2010). Through genetic knockout studies in mice, the β2*-containing nAChRs are known to mediate more affective vs somatic symptoms of nicotine withdrawal (Jackson et al, 2008). In humans, growing evidence also suggests that β2*-selective ligands could modify psychological withdrawal symptoms, which may influence long-term tobacco cessation.
Varenicline (Chantix), a partial agonist at α4β2* nAChRs, is now considered to be one of the leading medications for the enhancement of long-term smoking cessation. Mechanisms of varenicline that influence smoking cessation are, at least in part, through the reduction of affective components of withdrawal after long-term treatment (West et al, 2008). Over 13 million people have been prescribed varenicline, with many controlled studies demonstrating efficacy for assisting smokers in initiating and maintaining smoking abstinence and reducing psychological withdrawal (Cahill et al, 2011). The current commonly used treatment strategy is to administer varenicline starting at 0.5 mg per day and gradually increase the dose to 1 mg twice daily for the remaining treatment period (Tsai et al, 2007). When used as a treatment, low-dose varenicline (0.5 mg bid) demonstrated a two-fold increase (vs placebo) in tobacco cessation rates at 52-week follow-up, and had fewer adverse side effects than the higher dose (Cahill et al, 2011). Furthermore, varenicline treatment (1 mg bid or 1–4 0.5 mg ad lib) was shown to reduce affective withdrawal symptoms after 1–12 weeks of treatment in the studies reviewed by the Cochrane report, as evaluated through the Minnesota Nicotine Withdrawal Scale (MNWS) and the Questionnaire of Smoking Urges-Brief (QSU-brief) (Cahill et al, 2011). Varenicline treatment at higher (1 mg twice daily) vs lower doses is reported to have enhanced reductions in tobacco withdrawal symptoms (Cahill et al, 2011).
The main purpose of the study was to determine the degree of occupancy caused by varenicline and to measure the effective KD. It has previously been demonstrated that withdrawal symptoms do not respond to a single dose of varenicline but require chronic treatment. Thus, the current paper aims to determine whether in fact low-dose varenicline at the 0.5 mg dose actually binds to α4β2* nAChRs in humans and whether the effect is maximal, ie, by virtue of nearly full saturation, as predicted from preclinical studies (Rollema et al, 2010). Our findings demonstrate that even near-saturation of the receptors does not alleviate withdrawal symptoms. Results may better identify the pharmacological actions of varenicline in the human brain, which may (in the future) translate to better treatment and dosing regimens. We used positron emission tomography (PET) coupled with the selective α4β2* radioligand, 2-[(18)F]fluoro-A-85380 (2-FA), widely used by our laboratory and others (for a review on the fundamentals of the method, please see Lotfipour et al (2011b)). Through a double-blind, placebo-controlled study, we assessed 2-FA distribution volumes in the brain using a within-subject design (ie, the same participants were given a 0.5 mg dose of varenicline and matching pill placebo in separate PET sessions). We assessed the influence of varenicline, pill placebo, and smoking-to-satiety on anxiety and psychological withdrawal symptoms.
SUBJECTS AND METHODS
Participants and Ethical Approval
Research participants were recruited through advertisements on the internet on Craigslist. Study inclusion criteria were smoking ⩾10 cigarettes/day, 18–65 years of age, no previous history of psychiatric illness, no history of drug or alcohol abuse/dependence, and no metal body implants. The exclusion criteria for past psychiatric disorder/drug abuse was a self-report assessed through an in-person interview with the study principal investigator. Each participant was compensated $20/hour for time spent on study activities, and $100 to remain abstinent for two days before PET scanning. This study was approved by the Institutional Review Board for the VA Greater Los Angeles Medical Center.
Study Design
After obtaining informed consent, participants underwent an initial interview in which study inclusion/exclusion criteria were verified. Participants were asked to complete a set of behavioral rating scales on screening day and were asked to abstain from smoking for 2 nights before the testing session. We chose 2 nights of abstinence, based on our previous results that even the low plasma-nicotine level of 0.2 ng/ml (equivalent to a single puff on a cigarette) would occupy 20% of available nicotinic receptors (Brody et al, 2006). As a heavy smoker has plasma levels of nicotine equal to 50–100 ng/ml, with a half-life of 2.5 h of nicotine in the body (Benowitz and Jacob, 1993), 1 day of tobacco abstinence would equal nearly 10 half-lives leading to a plasma-nicotine level of ∼0.08–0.16 ng/ml. Thus, to obviate these confounds, we requested smokers to remain abstinent for 48 hours. Abstinence was later verified on the day before and the day of the testing session through carbon monoxide (CO) measures. On the day of the testing session, after confirmation of smoking abstinence, participants were required to test negative for dependent drugs and pregnancy (for females of child-bearing potential). At 0900 hours, participants were administered either varenicline or pill placebo in a randomized order (double-blind) and allowed to rest until 1200 hours.
At noon, participants were given intravenous bolus (3.8 mCi) plus continuous infusion of 2-FA (0.27 mCi/h). 2-FA was synthesized as described previously (Doll et al, 1999), and the bolus plus infusion was designed to produce an approximate steady-state level of 2-FA in the brain within 4 h after injection (Brody et al, 2006, 2009, 2011). Lunch was provided after infusion initiation and participants continued to rest until the first PET imaging block at 1600 hours.
Scanning sessions were performed in three separate blocks, the first lasting 60 min (block 1, 1600–1700 hours), followed by 40 min (block 2: 1730–1810 hours) and 50 min (block 3: 1830–1920 hours) block. Between block 1 and 2 (1700–1730 PM), participants smoked to satiety. During the smoking-to-satiety session, participants were advised: ‘you will have a 10-minute break to stand up, stretch outside of the scanner and smoke 2–3 cigarettes. You can smoke until you no longer need another cigarette.’
PET images were acquired using a Philips Gemini TF PET-CT scanner (Philips Healthcare, Eindhoven, the Netherlands). Data were acquired in fully 3-dimensional mode and reconstructed in 10-min frames using Fourier Rebinning and Filtered Back Projection. Attenuation correction was performed using the CT scan. Slice thickness was 0.2 cm and transaxial resolution 5.1 mm (full-width-half-maximum).
Placebo and Varenicline Administration
In a double-blind, placebo-controlled manner, each participant was administered either a placebo or low-dose varenicline (0.5 mg) pill, before the PET sessions, which were separated by at least 2 weeks. The timing of varenicline administration was chosen to have peak plasma levels at roughly the start of the 2-FA infusion at 1200 hours (Obach et al, 2006). The aim was to have peak occupancy of α4β2* nAChRs by varenicline at approximately the time of bolus 2-FA administration. This schedule allows 2-FA to reach steady-state levels in the presence of varenicline near its peak level. Given the long plasma half-life (17 h) of varenicline, we predicted high plasma levels of the medication throughout the 2-FA bolus plus infusion injection, thereby remaining reasonably constant during the initial period of 2-FA uptake (1200 to 1600 hours) and first block of the PET scanning session (1600–1700 hours) and slowly decreasing through the end of the imaging session (Obach et al, 2006).
Carbon Monoxide, Nicotine/Cotinine, and Toxicological Measurements
Exhaled carbon monoxide measures were obtained from participants during the initial screen, with an inclusion criteria of ⩾8 parts per million (ppm) for study enrollment (to verify smoking status). For each PET session, participants were asked to abstain from cigarette smoking for 2 days. Abstinence was tested through carbon monoxide measures (⩽4 ppm) and later verified with plasma nicotine (<1.0 ng/ml, the limit of detection) and cotinine analysis (<60 ng/ml). Carbon monoxide measures were obtained at 0900 hours (before placebo/varenicline administration, T1), 1200 hours (time of 2-FA infusion, T2), 1700 hours (before smoking, T3), 1730 hours (after smoking, T4), and at the end of PET scanning session, T5.
Blood Collection Times and Analysis
Blood-plasma samples were obtained for quantification of free, unmetabolized 2-FA, as previously reported (Brody et al, 2011), as well as for the measurement of nicotine, cotinine, and varenicline levels. Whole blood was collected in heparinized sealed tubes placed on ice until centrifugation; subsequently, plasma was collected and stored in polypropylene tubes. For 2-FA analysis, plasma was quantified on the day of the PET scan using methods previously published (Shumway et al, 2007; Sorger et al, 2006, 2007). For nicotine/cotinine and varenicline plasma analysis, samples were stored at −80 °C until the end of the study, and were shipped to and quantified by Pura Tech UCSF-Clinical Pharmacology Laboratory and Alta Analytical Laboratories, respectively.
Smokers Profile, Rating Scales
During the first screening session (Time 0, ie T0), participants completed the Smoker's Profile Form, Fagerström Test for Nicotine Dependence (Fagerstrom, 1978), Shiffman–Jarvik Withdrawal (Shiffman and Jarvik, 1976) Scale, Motivation for Smoking Scale (overall dependence score) (Russell et al, 1974), and BDI (Beck Depression Inventory) Rating Scale (Steer et al, 1999). Smokers were considered dependent if they smoked ⩾10 cigarettes/day, had a Fagerström Test for nicotine dependence score ⩾4, a Motivation for Smoking Scale (overall dependence score) ⩾6, and a Shiffman–Jarvik Withdrawal Scale ⩾3 per item. Participants also completed the State-Trait Anxiety Inventory (Spielberger, 1983), as well as the MNWS (Cappelleri et al, 2005; Hughes, 2007a; Hughes et al, 1991; Hughes and Hatsukami, 1986), QSU-brief (Cox et al, 2001), and SUTS (Strength of Urge to Smoke Scale) (Hughes, 2007a; Jarvik et al, 2000). The STAI, MNWS, QSU-brief, and the SUTS were subsequently administered at the five time points defined above, T1–T5, during each of the PET scanning sessions, to monitor craving and withdrawal symptoms during scanning.
Magnetic Resonance Imaging
Magnetic resonance imaging was performed within a week of the first PET session using a 1.5-T Magnetom Sonata scanner (Siemens AG, Erlangen, Germany). The specifications of the imaging sessions are: 3-dimensional Fourier-transform spoiled-gradient-recalled acquisition with a repetition time of 30 ms, an echo time of 7 ms, a 30° flip angle, two acquisitions, and a 256 × 192 view matrix. The volumes were reconstructed in 90 contiguous 1.5-mm-thick transaxial slices.
PET Analysis
PET data analysis, structural MRI co-registration, and motion correction were performed using PMOD, version 3.0 (PMOD Technologies, Zurich, Switzerland). Following the alignment of all blocks for both PET imaging scans with the structural MRI, regions of interest were drawn on the co-registered PET scans. Regions of interest were the right and left thalami, right and left middle frontal gyri, brainstem, cerebellum, and corpus callosum. The right and left thalami were drawn on approximately 7 slices, right and left middle frontal gyri on 1 slice, brainstem on 21 slices, cerebellum on 8 slices, and corpus callosum on 1 slice.
We calculated decay-corrected time-activity curves for each block (60, 40, and 50 min) of PET imaging and divided PET activities by plasma-free-2-FA activities to obtain free-fraction-corrected volumes of distribution, V/fP, using nomenclature derived from Innis et al (2007). We denote the binding volume of distribution for the placebo scan before smoking to satiety (Block 1) as VT/fP, and that for the varenicline scan before smoking to satiety as VV/fP. We denote the binding volume of distribution for the placebo scan after smoking to satiety (Block 3) as VC/fP and that for the varenicline scan after smoking to satiety as VVC/fP. Subjects were asked to smoke to satiety so that nicotine would displace nearly all of the specifically bound radioactive ligand. The block 3 scan gives a measurement of nonspecifically bound ligand, and can be subtracted from the block 1 and 2 scans to yield measurements of specific binding. We estimated the non-displaceable binding volume of distribution as VND/fP=VVC/fP, as these were the smallest volumes of distributions across all brain regions and no significant reductions were observed after smoking to satiety during the varenicline sessions. Therefore, the distribution volume for specific binding was estimated as VS/fP=VT/fP−VVC/fP. We calculated fractional receptor occupancy by the single low dose of varenicline from the following formula: BV/Bmax=(VT/fP−VV/fP)/(VS/fP)=(CV/KV)/(1+CV/KV). The dissociation constant of varenicline (KV) was determined using the mean concentration (CV) of varenicline tartrate, molecular weight 361.35 g/mol, in blood plasma. We also calculated fractional receptor occupancy due to smoking to satiety during the placebo session using BC/Bmax=(VT/fP–VC/fP)/(VS/fP), where BC is the density of receptors occupied due to smoking.
Statistical Analysis
We used Hotelling T-Square (multivariate Student t test) to assess the effect of medication (varenicline vs placebo) on distribution volume (VV/fP vs VT/fP) for all brain regions, followed by one-tail t-tests for individual regions. We similarly assessed the effect of smoking in the placebo session (VC/fP vs VT/fP) and in the varenicline session (VVC/fP vs VV/fP). To maintain adequate power, we grouped the higher binding-potential (BP) regions (thalamus, brainstem, cerebellum) separately from the lower BP regions (middle frontal gyrus and corpus callosum). The critical value for significance was set at a=0.05. The multivariate analysis presented determines the significant main effects of medication and smoking and significant interaction without the requirement of the post-hoc multiple-comparisons corrections required for an ANOVA analysis.
We analyzed the withdrawal rating scales MNWS, QSU-B, and SUTS for the effect of 2 days of withdrawal from smoking by Hotelling T-Square followed by post-hoc one-tail t-tests. We analyzed the STAI scale for effects of withdrawal, medication, and smoking using t-tests. All analyses were performed using MATLAB and JMP 9.0.2 statistical software (SAS Institute, Cary, NC, USA).
RESULTS
Enrollment
A total of nine subjects were included in the behavioral analysis with six subjects retained for the brain imaging analysis. For PET imaging, data from two subjects were excluded due to noncompliance with the protocol and/or inconsistency in the PET scans. A third subject was excluded due to the presence of a radiotracer-avid abnormal intraventricular mass that appeared to have resulted in abnormally low activities in brain. Two of the three participants excluded also had plasma-nicotine levels higher than 1.0 ng/ml, demonstrating that participants were probably not abstinent for at least the last 24 h. The subject with the presence of the intraventricular mass had 1.1 ng/ml plasma-nicotine levels on both placebo and varenicline PET scans, and the second subject had a 1.2 ng/ml plasma-nicotine level on his placebo scan. However, all participants were included in the behavioral analysis, as their CO measures were under 4 ppm on the testing day, indicating low levels of recent smoking.
Demographics and Initial Rating Scale Data in Comparison with Scan Days
The participant group was 67% male, mean age 38.2 (±3.4) years, 78% self-reported Caucasian and 22% self-reported non-Caucasian, and had a mean 14.8 (±0.5) years of education. They were moderately dependent smokers (16.7±1.7 cigarettes/day, FTND scores of 4.0±0.8, Motivation for Smoking Scale (overall dependence score) of 5.7 (±0.5)). On the screening day (without smoking abstinence), participants had minimal levels of depression (mean BDI score of 1.6 (±0.7)), and moderate levels of withdrawal, assessed using the Shiffman–Jarvik Withdrawal Scale with a score of 3.2 (±0.2) per item.
To determine whether withdrawal or anxiety rating scales increased due to 2 days of abstinence, we compared screening-day values (T0) to PET-imaging-day values (T1). Hotelling T-square for MNWS, QSU-B, and SUTS was not significant for either the placebo or varenicline days. The one-tail t-test comparing STAI values yielded p=0.03 for the placebo day and 0.22 for the varenicline day. Taking into account multiple comparisons, this result is non-significant.
Biochemical Measures
In all participants, CO measurements on the screening day confirmed the smoking habit with average exhaled levels of 11.8. (±1.1) ppm. Smoking abstinence was further identified through CO measurements on PET imaging days, with average values of 1.6 (±0.2) ppm at 0900 hours. Participants included in the PET-imaging data analysis had plasma nicotine levels that were below 1.0 ng/ml with low cotinine levels (56.81±8.1 ng/ml) on the day of PET imaging, supporting the patient reports of smoking abstinence. Smoking to satiety (average of 1.8±0.2 cigarettes) increased CO measures from before (1.6±0.2) to after smoking (6.3±0.8), p<0.0001, t-test. The number of cigarettes smoked during the varenicline (1.7±0.2) and placebo (1.9±0.2) scans did not significantly differ. The number of cigarettes smoked to satiety in this study was lower than in our earlier study (average of 2.8 cigarettes) (Brody et al, 2006), most likely due to different recruitment criteria in the current study for the number of cigarettes smoked per day: ⩾10 vs >20 cigarettes/day for the earlier study. The mean plasma varenicline level 3 h after oral administration was 1.7 (±0.1) ng/ml, and significantly decreased by the end of the scanning day (1.1±0.1 ng/ml, p<0.0001, t-test).
Rating Scales Assessed During the PET Session
The Hotelling T-square test comparing MNWS, QSU-B, and SUTS for the effect of medication at corresponding time points of the placebo and varenicline sessions was nonsignificant, meaning that withdrawal or craving symptoms did not differ between placebo and varenicline administration sessions. The Hotelling T-square test comparing these scales at points T3 (before smoking) and T4 (after smoking) were significant for both the placebo scan (p=0.01) and the varenicline scan (p=0.02). Post-hoc one-tail t-tests yielded P values of 0.08, 0.0007, 0.001 (0.19, 0.003, 0.004, excluding the subjects with >1.0 ng/ml of plasma nicotine levels) for the placebo scan and 0.011, 0.0003, 0.001 (0.02, 0.0014, 0.0019, excluding the subjects with >1.0 ng/ml of plasma nicotine levels) for the varenicline scan for MNWS, QSU-B, and SUTS, respectively. The t-tests comparing STAI at corresponding time points for the effect of medication were nonsignificant as were the t-tests comparing points T3 and T4 for both placebo and varenicline scans. Taken together, the results demonstrate that smoking to satiety significantly reduced withdrawal symptoms in both placebo and varenicline treatment groups equally, whereas the measure of anxiety was not affected. Testing for a main effect of medication separately per question, our findings demonstrate that varenicline administration does not reduce any aspects of withdrawal for any questions asked at the time points assessed (T1–5). Our findings provide evidence that an acute exposure to low-dose varenicline does not reduce the psychological symptoms of withdrawal as assessed through the MNWS, QSU-B, and SUTS.
PET Findings
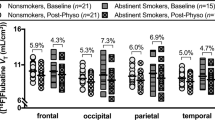
Time series data (across subjects) are shown for the thalamus (Figure 1), the brain region with the highest density of nAChRs and 2-FA radioactivity counts (Brody et al, 2006). Mean values (across subject) of VT/fP, VS/fP, BV/Bmax, and BC/Bmax for each brain region are given in Tables 1 and 2.
Time-activity curves for positron emission tomography (PET) data for averaged values of the right and left thalamus. Participants underwent two PET scanning sessions, and were administered either placebo or 0.5 mg of varenicline (double-blind, randomized order) at 0900 hours of the two PET sessions. At 1200 hours, the α4β2* radioligand, 2-[ (18)F]fluoro-A-85380 (2-FA) was given as a bolus (3.8 mCi) plus infusion injection of 2-FA (0.27 mCi/h), reaching steady-state levels over 4 h. At 1600 hours, participants underwent 60 min of PET imaging. Following this imaging, participants smoked to satiety, followed by two additional PET imaging sessions (40 and 50 min).
The Hotelling T-square demonstrated a significant effect of medication on BP, by comparing VT/fP and VV/fP values, in both the grouped high-BP and low-BP regions (Tables 1 and 2, Figures 1 and 2). Post-hoc one-tailed t-tests were significant for all brain regions. For the placebo session, we found a significant effect of smoking to satiety on BP by comparing VT/fP and VC/fP for both sets of regions. Post-hoc one-tailed t-tests were again significant for all brain regions. Comparing VV/fP with VVC/fP (varenicline session), we find a P-value of 0.05 for smoking-to-satiety for the high-BP regions, but no significance for the low-BP regions. We have Bonferroni-corrected significance only for the thalamic regions. These data indicate that smoking to satiety following varenicline was minimally significant in reducing VV/fP values, and only observable in the thalamus (Figure 1).
Representative positron emission tomography (PET) images of a smoker after placebo or varenicline administration before and after smoking. Images were obtained during the first hour of scanning before smoking to satiety and 10 min after smoking to satiety. The images illustrate the significant reduction of 2-FA availability particularly in the thalamus, a region known to have the highest density of nicotinic receptors in the human brain. A magnetic resonance imaging (MRI) scan used for co-registration is included.
The preceding analysis indicates that percent occupancies for varenicline (0.5 mg administered orally) are compatible with 100% occupancy. To further characterize this result, we set 90% lower confidence limits for each brain region, given in Tables 1 and 2. These vary from approximately 90% occupancy for thalamus and brainstem to about 75% occupancy for middle frontal gyri and corpus callosum.
From these values and measurements of plasma varenicline concentration, we set 90% upper limits on KV, the dissociation constant for varenicline binding, for the high BP regions, with respect to plasma varenicline concentration. Based on in-vivo measurements of free drug exposures in the brain, the extracellular brain concentration is expected to be nearly identical to the plasma concentration (Rollema et al, 2010). Thus, our upper limit is relevant to the true value of KV. Using the mean 90% lower limit for BV/Bmax of 89% and the mean plasma varenicline concentration for these subjects of 1.4 ng/ml, we have KV⩽0.18 ng/ml (90%) or equivalently KV<0.49 nM (90%). This value is compatible with published in-vitro values for human cortex (0.15 nM) and human α4β2* nAChRs in HEK cells (0.11 nM) (Rollema et al, 2007). The percentage occupancies by brain region for smoking to satiety during the placebo scan are also given in Tables 1 and 2. These values are approximately 90%, corresponding to smoking a mean of 1.8 cigarettes, and are compatible with our previously published analysis (Brody et al, 2006). Fractional occupancies >1.0 reflect measurement uncertainty.
As nearly complete occupancy of α4β2* nAChRs by varenicline does not alleviate withdrawal symptoms, but smoking to satiety in the presence of full occupancy does, the data suggest that smoking reduces withdrawal symptoms by mechanisms at least partly independent of α4β2* nAChRs.
DISCUSSION
Our findings demonstrate that a single low dose of varenicline saturates (>90% occupancy) α4β2* nAChRs in the human brain. However, this dose of varenicline did not significantly reduce withdrawal symptoms. Compatible with our earlier study (Brody et al, 2006), smoking to satiety results in >90% occupancy of available nAChRs and significantly reduces withdrawal symptoms in a placebo PET session. Furthermore, these data suggest that smoking to satiety reduces withdrawal symptoms through mechanisms both including and independent of α4β2* nAChR occupancy. It is well known that the success of varenicline in smoking cessation requires long-term treatment. We speculate that this effect may be mediated by the putative increase in nicotinic receptor density levels or some other receptor-related mechanism.
The Role of Varenicline in the Mechanisms Reducing Tobacco Withdrawal
Given previous preclinical findings that α4β2* nAChRs do not influence somatic withdrawal, our current analysis focused primarily on psychological components of withdrawal, as assessed through the QSU-Brief, S-UTS, and Minnessota Nicotine Withdrawal Rating Scales. Low-dose varenicline saturated the majority of nAChRs without influencing psychological withdrawal symptoms. Other studies have demonstrated a significant reduction of withdrawal symptoms after multiple weeks of varenicline administration (with peak reduction of withdrawal symptoms observed after averaging 6 weeks of scores), using similar withdrawal ratings scales as in our current study (Tsai et al, 2007). In this study, given the saturation of α4β2* nAChRs by low-dose varenicline, its inability to reduce withdrawal symptoms after a single administration, confirms the importance of long-term administration to achieve withdrawal alleviation. Chronic effects of varenicline have recently been reported in the brain imaging literature, with findings demonstrating that as little as 3 weeks of treatment can reduce cue-associated brain reactivity in limbic reward regions in healthy non-abstinent smokers (Franklin et al, 2011). Thus, the findings suggest that visual ‘cues’ lead to brain activity (blood-oxygen-level dependence (BOLD) response) through an α4β2* nAChR mechanism, which can be blocked by varenicline. Whether other sensory cues, such as smell, taste, and feel of a cigarette, could work through similar mechanisms needs further exploration. Such effects may be driven by chronic varenicline-mediated modifications of the α4β2* nAChR densities in the brain (Franklin et al, 2011). Indeed, preclinical evidence demonstrates that chronic varenicline treatment can increase the density of α4β2* nAChRs after a 2-week administration (with behavioral correlates), similar to the effects of chronic nicotine exposure over the same time period (Turner et al, 2011). Thus, our findings provide rationale to assess whether chronic administration of varenicline can reduce psychological withdrawal symptoms through the modulation of the density of α4β2* nAChR. As we have shown that full-occupancy α4β2* binding of varenicline does not reduce withdrawal symptoms, the remaining question is what does modulate withdrawal symptoms. It is possible that a downstream effect that is directly due to long-term binding of the α4β2* receptors may be important, or perhaps something more remote. It should be noted, however, that acute exposure to varenicline (0.1 mg/kg) can reduce withdrawal severity in animal models, particularly when measuring memory deficits (Raybuck et al, 2008), although the dose is higher than that used in our current study, not taking into account pharmacokinetic differences between rodent and human species. Therefore, the assessment of memory deficits and other measures, including negative affect, mood, and general cognition, would be beneficial to include in future studies to evaluate whether all aspects of withdrawal severity are unaffected by a single oral dose of varenicline. Indeed, chronic administration of varenicline can improve mood and cognition during abstinence (Patterson et al, 2009) as well as working memory (Loughead et al, 2010), and reduce negative affect (West et al, 2008) in smokers during abstinence. Such studies would help clarify the critical components of the withdrawal syndrome that varenicline targets to mediate its therapeutic effects.
Our findings have implications relating to the length of the pharmacological treatment regimen necessary to influence withdrawal severity. Repeated exposure to varenicline at the low or a higher dose will ultimately reduce withdrawal symptoms (Cahill et al, 2011), most likely through the actions on α4β2* nAChRs. By further assessing varenicline's actions at its drug target, results may aid future studies in designing individually tailored treatment regimens necessary to reduce withdrawal severity and induce smoking cessation. Also, studies may promote the use of more specific therapeutic targets for mediating smoking cessation. Such studies are important, as even subtle changes in the nAChR assembly (eg, through the addition of the α5 subunit to the α4β2 receptor complex) can influence the pharmacological properties of these receptors (Ramirez-Latorre et al, 1996). Designing improved treatment regimens and drugs targeting specific nAChR complexes may significantly assist in smoking-cessation-treatment efficacy in the future.
Varenicline Pharmacokinetic and Pharmacodynamic Mechanisms Influencing Tobacco Withdrawal
The study of higher dose and prolonged regimens of varenicline treatment is required for understanding of the pharmacokinetic and pharmacodynamic mechanisms that influence tobacco withdrawal symptoms and smoking cessation. Our data show that single low-dose (0.5 mg) administration produces a 3–5 nM (1.1–1.7 ng/ml) concentration of varenicline in human blood plasma, which lead to complete saturation of α4β2* nAChRs in the human brain. This concentration of varenicline is lower than the reported therapeutic window (32–131 nM) in mediating smoking cessation using standard dosing regimens (Rollema et al, 2010). Indeed, it is interesting to note that the 32–131 nM concentrations of varenicline desensitize human α4β2* nAChRs to repeated administrations of acetylcholine in oocyte electrophysiological studies (IC50=0.07 nM) (Rollema et al, 2010). A single low dose of varenicline leads to a plasma level below the window that induces the desensitization response and the latter may be required to mediate a behavioral effect. Why desensitization and behavioral effects require higher levels than those required for complete saturation of α4β2* nAChRs after low-dose administration of varenicline is not known and needs further investigation. Taken together with our previous findings that humans continue to smoke in the presence of complete saturation of α4β2* nAChRs after one cigarette (Brody et al, 2006), the data suggest that non-α4β2* nAChR subtypes (or other mechanisms) may have a role in nicotine addiction, in the reduction of varenicline-induced tobacco withdrawal severity, as well as in the induction of smoking cessation. Future studies examining standard treatment regimens, larger study populations, and radioligands for non-α4β2* nAChRs are needed to further identify the mechanisms mediating varenicline-induced smoking cessation.
The Role of Non-α4β2* Nicotinic Receptor in Mediating Tobacco Withdrawal
Our data reveal that, although varenicline saturates α4β2* nAChRs, cigarette smoking induced a reduction of withdrawal severity in varenicline-treated subjects in the absence of further displacement of 2-FA for almost all brain regions. We speculate that cigarette smoking may reduce withdrawal severity in varenicline-treated subjects through mechanisms independent of α4β2* nAChRs. Indeed, a number of neuronal nAChR subunits have been discovered, including alpha (α2–7, 9, 10) and beta (β2–4) subunits, and a large body of evidence exists for the role of non-α4β2* nicotinic receptor subunits in mediating withdrawal (Changeux, 2010). Systemic nAChR antagonists can precipitate withdrawal in mice chronically treated with nicotine, with genetic animal models demonstrating an absence (or significant reductions) of somatic (physical) signs of withdrawal in α2, α5, or β4 null mutant mice (Changeux, 2010). In contrast, somatic, but not affective (psychological), signs were present in homozygous β2 null mutant mice (Jackson et al, 2008) (similar to the role of α6-containing nicotinic receptors as evaluated through pharmacological studies (Jackson et al, 2009)). The findings suggest that certain nAChR subunits, but not others, differentially regulate somatic and affective nicotine-withdrawal symptoms. Thus, both clinical and preclinical evidence suggest that non-α4β2* nAChRs are important contributors to tobacco and general drug addiction. Such mechanisms may work separately or in parallel with the 4000 + constituents in tobacco smoke (eg, norharmane, harmane, or other monoamine oxidase inhibitors (Lotfipour et al, 2011a)) to influence tobacco-withdrawal symptoms in the human smoker. Whether smoking to satiety decreases withdrawal severity through non-α4β2* nAChRs needs further assessment.
Significance of our Studies Based on the Functional Selectivity of 2-FA at High-Affinity α4β2* nAChRs
Researchers have identified a differential-state model for nAChRs (ie, resting (R), active (A), intermediate (I), desensitized (D), as discussed by Quick and Lester (2002). The high-affinity α4β2* nAChRs have been modeled, eg, in oocyte-expression systems, by modifying the ratios of the number of α4 to β2 subunits within the system (Moroni et al, 2006) or genetically (Harpsoe et al, 2011). Evidence suggests that A-85380 is more functional at the high-affinity nAChRs that have (α4)(2)(β2)(3) vs the low-affinity site having (α4)(3)(β2)(2) stoichiometry (Moroni et al, 2006). These receptor subtypes can have differing functionality, depending, eg, on the length of nicotine exposure (Sokolova et al, 2005) and the presence or absence of excess α4 subunits (Harpsoe et al, 2011) or accessory subunits (such as α5) (Ramirez-Latorre et al, 1996). Unlabeled A-85830 and nicotine, but less so varenicline, appear to have higher functional efficacy at (α4)(2)(β2)(3) vs (α4)(3)(β2)(2) stoichiometry nAChRs (Anderson et al, 2009; Moroni et al, 2006). How the activation and desensitization properties of these nAChRs are modified in the human smoker is not known; although it has been hypothesized that both receptor properties could influence nicotine addiction and mood (Picciotto et al, 2008). Although some authors have provided evidence to suggest that upregulated high-affinity nAChRs may be nonfunctional (Quick and Lester, 2002), other evidence suggests that upregulated high-affinity nAChRs retain functionality in the presence of chronic nicotine (Sokolova et al, 2005), and under certain circumstances are less sensitive to desensitization (Buisson and Bertrand, 2001). The possible reasons for the discrepancy between the findings have been discussed previously (Quick and Lester, 2002). In the living human brain, whether high-affinity upregulated nAChRs that bind to varenicline, nicotine, and 2-FA remain functional needs further evaluation.
Limitations
Our findings demonstrate that 2 days of withdrawal from cigarettes did not significantly influence withdrawal symptoms. The lack of significance may be due to the small sample size. However the research setting, where participants were paid for staying abstinent, and were informed at the onset of the study that they could smoke 1 h after the PET imaging session, may have had a role. Such factors may interact concomitantly to reduce the overall intensity of psychological withdrawal severity and need to be taken into consideration in future studies (Hughes, 2007b). We are limited in that our tracer is selective for α4β2* nAChRs, so that we are unable to associate other nicotinic receptors with anxiety and withdrawal rating scales. Furthermore, the uncertainty in our measurement of BP is determined by the region size and density of α4β2* nAChRs. The thalamic BP is by far the best determined. We are sensitive to incremental binding of α4β2* nAChRs by nicotine in thalamus but not to BP changes in other regions where the measurement uncertainty in BP is greater. Such factors need to be taken into consideration when interpreting findings from our current study.
CONCLUSIONS
Our findings demonstrate that a low dose of varenicline saturates α4β2* nAChRs in the human brain. A single low dose of varenicline did not produce a reduction of withdrawal severity, as was observed when participants smoked to satiety. Furthermore, cigarette smoking reduced withdrawal severity in the presence of complete saturation of α4β2* by varenicline. The findings highlight the possibility that cigarette smoking reduces withdrawal symptoms through mechanisms independent of (or in addition to) α4β2* nAChR occupancy. Future longitudinal studies with larger study populations are needed to assess the impact of low- and standard-dose varenicline on nAChR receptor densities and occupancy in relation to cigarette smoking reward and withdrawal. Results from such studies would assist in better understanding the mechanism mediating varenicline-induced smoking cessation, and may help develop improved medications for the induction of smoking abstinence in the future.
References
Anderson DJ, Malysz J, Gronlien JH, El Kouhen R, Hakerud M, Wetterstrand C et al (2009). Stimulation of dopamine release by nicotinic acetylcholine receptor ligands in rat brain slices correlates with the profile of high, but not low, sensitivity alpha4beta2 subunit combination. Biochem Pharmacol 78: 844–851.
Benowitz NL, Jacob III P (1993). Nicotine and cotinine elimination pharmacokinetics in smokers and nonsmokers. Clin Pharmacol Ther 53: 316–323.
Brody AL, Mandelkern MA, Costello MR, Abrams AL, Scheibal D, Farahi J et al (2009). Brain nicotinic acetylcholine receptor occupancy: effect of smoking a denicotinized cigarette. Int J Neuropsychopharmacol 12: 305–316.
Brody AL, Mandelkern MA, London ED, Khan A, Kozman D, Costello MR et al (2011). Effect of secondhand smoke on occupancy of nicotinic acetylcholine receptors in brain. Arch Gen Psychiatry 68: 953–960.
Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D et al (2006). Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch Gen Psychiatry 63: 907–915.
Buisson B, Bertrand D (2001). Chronic exposure to nicotine upregulates the human (alpha)4((beta)2 nicotinic acetylcholine receptor function. J Neurosci 21: 1819–1829.
Cahill K, Stead LF, Lancaster T (2011). Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev(2): CD006103.
Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG (2005). Revealing the multidimensional framework of the Minnesota nicotine withdrawal scale. Curr Med Res Opin 21: 749–760.
Changeux JP (2010). Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat Rev Neurosci 11: 389–401.
Cox LS, Tiffany ST, Christen AG (2001). Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res 3: 7–16.
Doll F, Dolci L, Valette H, Hinnen F, Vaufrey F, Guenther I et al (1999). Synthesis and nicotinic acetylcholine receptor in vivo binding properties of 2-fluoro-3-[2(S)-2-azetidinylmethoxy]pyridine: a new positron emission tomography ligand for nicotinic receptors. J Med Chem 42: 2251–2259.
Fagerstrom KO (1978). Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav 3: 235–241.
Fowler CD, Arends MA, Kenny PJ (2008). Subtypes of nicotinic acetylcholine receptors in nicotine reward, dependence, and withdrawal: evidence from genetically modified mice. Behav Pharmacol 19: 461–484.
Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y et al (2011). Effects of varenicline on smoking cue-triggered neural and craving responses. Arch Gen Psychiatry 68: 516–526.
Harpsoe K, Ahring PK, Christensen JK, Jensen ML, Peters D, Balle T (2011). Unraveling the high- and low-sensitivity agonist responses of nicotinic acetylcholine receptors. J Neurosci 31: 10759–10766.
Hatsukami D, McBride C, Pirie P, Hellerstedt W, Lando H (1991). Effects of nicotine gum on prevalence and severity of withdrawal in female cigarette smokers. J Subst Abuse 3: 427–440.
Hughes JR (2007a). Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res 9: 315–327.
Hughes JR (2007b). Measurement of the effects of abstinence from tobacco: a qualitative review. Psychol Addict Behav 21: 127–137.
Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW (1991). Symptoms of tobacco withdrawal. A replication and extension. Arch Gen Psychiatry 48: 52–59.
Hughes JR, Hatsukami D (1986). Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry 43: 289–294.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN et al (2007). Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27: 1533–1539.
Jackson KJ, Martin BR, Changeux JP, Damaj MI (2008). Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther 325: 302–312.
Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI (2009). The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. J Pharmacol Exp Ther 331: 547–554.
Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL (2000). Nicotine blood levels and subjective craving for cigarettes. Pharmacol Biochem Behav 66: 553–558.
Kenny PJ, Markou A (2001). Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav 70: 531–549.
Lotfipour S, Arnold MM, Hogenkamp DJ, Gee KW, Belluzzi JD, Leslie FM (2011a). The monoamine oxidase (MAO) inhibitor tranylcypromine enhances nicotine self-administration in rats through a mechanism independent of MAO inhibition. Neuropharmacology 61: 95–104.
Lotfipour S, Ferguson E, Leonard G, Perron M, Pike B, Richer L et al (2009). Orbitofrontal cortex and drug use during adolescence: role of prenatal exposure to maternal smoking and BDNF genotype. Arch Gen Psychiatry 66: 1244–1252.
Lotfipour S, Leonard G, Perron M, Pike B, Richer L, Seguin JR et al (2010). Prenatal exposure to maternal cigarette smoking interacts with a polymorphism in the alpha6 nicotinic acetylcholine receptor gene to influence drug use and striatum volume in adolescence. Mol Psychiatry 15: 6–8.
Lotfipour S, Mandelkern MA, Brody AL (2011b). Quantitative Molecular Imaging of Neuronal Nicotinic Acetylcholine Receptors in the Human Brain with A-85380 Radiotracers. Curr Med Imaging Rev 7: 107–112.
Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S et al (2010). Effects of the alpha4beta2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biol Psychiatry 67: 715–721.
Moroni M, Zwart R, Sher E, Cassels BK, Bermudez I (2006). alpha4beta2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol Pharmacol 70: 755–768.
Obach RS, Reed-Hagen AE, Krueger SS, Obach BJ, O’Connell TN, Zandi KS et al (2006). Metabolism and disposition of varenicline, a selective alpha4beta2 acetylcholine receptor partial agonist, in vivo and in vitro. Drug Metab Dispos 34: 121–130.
Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC et al (2009). Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry 65: 144–149.
Picciotto MR, Addy NA, Mineur YS, Brunzell DH (2008). It is not ‘either/or’: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol 84: 329–342.
Quick MW, Lester RA (2002). Desensitization of neuronal nicotinic receptors. J Neurobiol 53: 457–478.
Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L (1996). Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature 380: 347–351.
Raybuck JD, Portugal GS, Lerman C, Gould TJ (2008). Varenicline ameliorates nicotine withdrawal-induced learning deficits in C57BL/6 mice. Behav Neurosci 122: 1166–1171.
Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA et al (2007). Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52: 985–994.
Rollema H, Shrikhande A, Ward KM, Tingley III FD, Coe JW, O’Neill BT et al (2010). Pre-clinical properties of the alpha4beta2 nicotinic acetylcholine receptor partial agonists varenicline, cytisine and dianicline translate to clinical efficacy for nicotine dependence. Br J Pharmacol 160: 334–345.
Russell MA, Peto J, Patel UA (1974). The classification of smoking by factorial structure of motives. J R Stat Soc Ser A 137: 313–346.
Shiffman SM, Jarvik ME (1976). Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology 50: 35–39.
Shumway DA, Pavlova OA, Mukhin AG (2007). A simplified method for the measurement of nonmetabolized 2-[18F]F-A-85380 in blood plasma using solid-phase extraction. Nucl Med Biol 34: 221–228.
Sokolova E, Matteoni C, Nistri A (2005). Desensitization of neuronal nicotinic receptors of human neuroblastoma SH-SY5Y cells during short or long exposure to nicotine. Br J Pharmacol 146: 1087–1095.
Sorger D, Becker GA, Hauber K, Schildan A, Patt M, Birkenmeier G et al (2006). Binding properties of the cerebral alpha4beta2 nicotinic acetylcholine receptor ligand 2-[18F]fluoro-A-85380 to plasma proteins. Nucl Med Biol 33: 899–906.
Sorger D, Becker GA, Patt M, Schildan A, Grossmann U, Schliebs R et al (2007). Measurement of the alpha4beta2* nicotinic acetylcholine receptor ligand 2-[(18)F]Fluoro-A-85380 and its metabolites in human blood during PET investigation: a methodological study. Nucl Med Biol 34: 331–342.
Spielberger C (1983). Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press: Palo Alto, CA.
Steer RA, Cavalieri TA, Leonard DM, Beck AT (1999). Use of the Beck Depression Inventory for Primary Care to screen for major depression disorders. Gen Hosp Psychiatry 21: 106–111.
Toledo-Rodriguez M, Lotfipour S, Leonard G, Perron M, Richer L, Veillette S et al (2010). Maternal smoking during pregnancy is associated with epigenetic modifications of the brain-derived neurotrophic factor-6 exon in adolescent offspring. Am J Med Genet B Neuropsychiatr Genet 153B: 1350–1354.
Tsai ST, Cho HJ, Cheng HS, Kim CH, Hsueh KC, Billing Jr CB et al (2007). A randomized, placebo-controlled trial of varenicline, a selective alpha4beta2 nicotinic acetylcholine receptor partial agonist, as a new therapy for smoking cessation in Asian smokers. Clin Ther 29: 1027–1039.
Turner JR, Castellano LM, Blendy JA (2011). Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res 13: 41–46.
Ueta I, Saito Y, Teraoka K, Miura T, Jinno K (2010). Determination of volatile organic compounds for a systematic evaluation of third-hand smoking. Anal Sci 26: 569–574.
West R, Baker CL, Cappelleri JC, Bushmakin AG (2008). Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology 197: 371–377.
Acknowledgements
We thank Karen Ta, Jaime La Charite, Betram Nweke, Stuart Mirell, Van Cayetano, Chien Nguyen, Judah Farahi, Andrew Leuchter, Hans Rollema, Maziar Maghsoodnia, Alison Curtin, Ron Waldorf, Diep Seversen, and Josephine Ribe for their assistance on the study. This work was funded by Pfizer Pharmaceutical Corporation (Grant number: WS653344) (SL) and, in part, by T-32 NIMH post-doctoral fellowship (MH17140) (SL), NIDA (R01 DA20872) (ALB), and the Richard Metzner Chair in Clinical Neuropharmacology (ALB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that, except for income received from our primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Lotfipour, S., Mandelkern, M., Alvarez-Estrada, M. et al. A Single Administration of Low-Dose Varenicline Saturates α4β2* Nicotinic Acetylcholine Receptors in the Human Brain. Neuropsychopharmacol 37, 1738–1748 (2012). https://doi.org/10.1038/npp.2012.20
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2012.20
Keywords
This article is cited by
-
Bidirectional Associations among Nicotine and Tobacco Smoke, NeuroHIV, and Antiretroviral Therapy
Journal of Neuroimmune Pharmacology (2020)
-
Tobacco smoking and dopaminergic function in humans: a meta-analysis of molecular imaging studies
Psychopharmacology (2019)
-
Combination therapy of varenicline with nicotine replacement therapy is better than varenicline alone: a systematic review and meta-analysis of randomized controlled trials
BMC Public Health (2015)
-
Varenicline effects on drinking, craving and neural reward processing among non-treatment-seeking alcohol-dependent individuals
Psychopharmacology (2014)