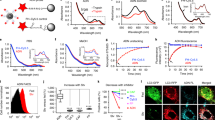
Abstract
Semiconductor quantum dots and superparamagnetic iron oxide nanocrystals have physical properties that are well suited for biomedical imaging. Previously, we have shown that iron oxide nanocrystals embedded within the lipid core of micelles show optimized characteristics for quantitative imaging. Here, we embed quantum dots and superparamagnetic iron oxide nanocrystals in the core of lipoproteins—micelles that transport lipids and other hydrophobic substances in the blood—and show that it is possible to image and quantify the kinetics of lipoprotein metabolism in vivo using fluorescence and dynamic magnetic resonance imaging. The lipoproteins were taken up by liver cells in wild-type mice and displayed defective clearance in knock-out mice lacking a lipoprotein receptor or its ligand, indicating that the nanocrystals did not influence the specificity of the metabolic process. Using this strategy it is possible to study the clearance of lipoproteins in metabolic disorders and to improve the contrast in clinical imaging.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sosnovik, D. E., Nahrendorf, M. & Weissleder, R. Molecular magnetic resonance imaging in cardiovascular medicine. Circulation 115, 2076–2086 (2007).
Mulder, W. J., Strijkers, G. J., van Tilborg, G. A., Griffioen, A. W. & Nicolay, K. Lipid-based nanoparticles for contrast-enhanced MRI and molecular imaging. NMR Biomed. 19, 142–164 (2006).
Bowen, C. V., Zhang, X. W., Saab, G., Gareau, P. J. & Rutt, B. K. Application of the static dephasing regime theory to superparamagnetic iron-oxide loaded cells. Magn. Reson. Med. 48, 52–61 (2002).
Simon, G. H. et al. T1 and T2 relaxivity of intracellular and extracellular USPIO at 1.5T and 3T clinical MR scanning. Eur. Radiol. 16, 738–745 (2006).
Yablonskiy, D. A. & Haacke, E. M. Theory of NMR signal behavior in magnetically inhomogeneous tissues: the static dephasing regime. Magn. Reson. Med. 32, 749–763 (1994).
Tromsdorf, U. I. et al. Size and surface effects on the MRI relaxivity of manganese ferrite nanoparticle contrast agents. Nano Lett. 7, 2422–2427 (2007).
Alivisatos, P. The use of nanocrystals in biological detection. Nature Biotechnol. 22, 47–52 (2004).
Michalet, X. et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307, 538–544 (2005).
Bruchez, M., Jr, Moronne, M., Gin, P., Weiss, S. & Alivisatos, A. P. Semiconductor nanocrystals as fluorescent biological labels. Science 281, 2013–2016 (1998).
Chan, W. C. & Nie, S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 281, 2016–2018 (1998).
Dubertret, B. et al. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 298, 1759–1762 (2002).
Gao, X., Cui, Y., Levenson, R. M., Chung, L. W. & Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nature Biotechnol. 22, 969–976 (2004).
Kim, S. et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nature Biotechnol. 22, 93–97 (2004).
Bamba, V. & Rader, D. J. Obesity and atherogenic dyslipidemia. Gastroenterology 132, 2181–2190 (2007).
Glassberg, H. & Rader, D. J. Management of lipids in the prevention of cardiovascular events. Annu. Rev. Med. 59, 79–94 (2008).
Frias, J. C., Williams, K. J., Fisher, E. A. & Fayad, Z. A. Recombinant HDL-like nanoparticles: a specific contrast agent for MRI of atherosclerotic plaques. J. Am. Chem. Soc. 126, 16316–16317 (2004).
Corbin, I. R. et al. Low-density lipoprotein nanoparticles as magnetic resonance imaging contrast agents. Neoplasia 8, 488–498 (2006).
Glickson, J. D. et al. Lipoprotein nanoplatform for targeted delivery of diagnostic and therapeutic agents. Mol. Imaging 7, 101–110 (2008).
Penfield, J. G. & Reilly, R. F., Jr. What nephrologists need to know about gadolinium. Nat. Clin. Pract. Nephrol. 3, 654–668 (2007).
Karpe, F. Postprandial lipoprotein metabolism and atherosclerosis. J. Intern. Med. 246, 341–355 (1999).
Zheng, C., Murdoch, S. J., Brunzell, J. D. & Sacks, F. M. Lipoprotein lipase bound to apolipoprotein B lipoproteins accelerates clearance of postprandial lipoproteins in humans. Arterioscler. Thromb. Vasc. Biol. 26, 891–896 (2006).
Merkel, M., Eckel, R. H. & Goldberg, I. J. Lipoprotein lipase: genetics, lipid uptake and regulation. J. Lipid Res. 43, 1997–2006 (2002).
Heeren, J., Niemeier, A., Merkel, M. & Beisiegel, U. Endothelial-derived lipoprotein lipase is bound to postprandial triglyceride-rich lipoproteins and mediates their hepatic clearance in vivo. J. Mol. Med. 80, 576–584 (2002).
Beisiegel, U., Weber, W. & Bengtsson-Olivecrona, G. Lipoprotein lipase enhances the binding of chylomicrons to low density lipoprotein receptor-related protein. Proc. Natl Acad. Sci. USA 88, 8342–8346 (1991).
Mahley, R. W. & Ji, Z. S. Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J. Lipid Res. 40, 1–16 (1999).
Martins, I. J. & Redgrave, T. G. Obesity and post-prandial lipid metabolism. Feast or famine? J. Nutr. Biochem. 15, 130–141 (2004).
Chan, D. C., Barrett, P. H. & Watts, G. F. Recent studies of lipoprotein kinetics in the metabolic syndrome and related disorders. Curr. Opin. Lipidol. 17, 28–36 (2006).
Magkos, F. & Sidossis, L. S. Measuring very low density lipoprotein-triglyceride kinetics in man in vivo: how different the various methods really are. Curr. Opin. Clin. Nutr. Metab. Care 7, 547–555 (2004).
Havel, R. J. & Hamilton, R. L. Hepatic catabolism of remnant lipoproteins: where the action is. Arterioscler. Thromb. Vasc. Biol. 24, 213–215 (2004).
Hussain, M. M. et al. Chylomicron metabolism—chylomicron uptake by bone-marrow in different animal species. J. Biol. Chem. 264, 17931–17938 (1989).
Rensen, P. C. et al. Particle size determines the specificity of apolipoprotein E-containing triglyceride-rich emulsions for the LDL receptor versus hepatic remnant receptor in vivo. J. Lipid Res. 38, 1070–1084 (1997).
Saini, S. et al. Ferrite particles: a superparamagnetic MR contrast agent for the reticuloendothelial system. Radiology 162, 211–216 (1987).
de Rijke, Y. B., Hessels, E. M. & Van Berkel, T. J. Recognition sites on rat liver cells for oxidatively modified beta-very low density lipoproteins. Arterioscler. Thromb. 12, 41–49 (1992).
Gillis, P., Moiny, F. & Brooks, R. A. On T(2)-shortening by strongly magnetized spheres: a partial refocusing model. Magn. Reson. Med. 47, 257–263 (2002).
Brooks, R. A. T(2)-shortening by strongly magnetized spheres: a chemical exchange model. Magn. Reson. Med. 47, 388–391 (2002).
Lee, J. H. et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nature Med. 13, 95–99 (2007).
McAteer, M. A. et al. In vivo magnetic resonance imaging of acute brain inflammation using microparticles of iron oxide. Nature Med. 13, 1253–1258 (2007).
Shapiro, E. M. et al. MRI detection of single particles for cellular imaging. Proc. Natl Acad. Sci. USA 101, 10901–10906 (2004).
Tiret, L. et al. Postprandial response to a fat tolerance test in young adults with a paternal history of premature coronary heart disease—the EARS II study (European Atherosclerosis Research Study). Eur. J. Clin. Invest. 30, 578–585 (2000).
Aalto-Setala, K. et al. Mechanism of hypertriglyceridemia in human apolipoprotein (apo) CIII transgenic mice. Diminished very low density lipoprotein fractional catabolic rate associated with increased apo CIII and reduced apo E on the particles. J. Clin. Invest. 90, 1889–1900 (1992).
Merkel, M. et al. Catalytically inactive lipoprotein lipase expression in muscle of transgenic mice increases very low density lipoprotein uptake: direct evidence that lipoprotein lipase bridging occurs in vivo. Proc. Natl Acad. Sci. USA 95, 13841–13846 (1998).
Ishibashi, S., Herz, J., Maeda, N., Goldstein, J. L. & Brown, M. S. The 2-receptor model of lipoprotein clearance—tests of the hypothesis in knockout mice lacking the low-density-lipoprotein receptor, apolipoprotein-e, or both proteins. Proc. Natl Acad. Sci. USA 91, 4431–4435 (1994).
Herz, J. et al. Initial hepatic removal of chylomicron remnants is unaffected but endocytosis is delayed in mice lacking the low density lipoprotein receptor. Proc. Natl Acad. Sci. USA 92, 4611–4615 (1995).
Mortimer, B. C., Beveridge, D. J., Martins, I. J. & Redgrave, T. G. Intracellular localization and metabolism of chylomicron remnants in the livers of low density lipoprotein receptor-deficient mice and apoE-deficient mice. Evidence for slow metabolism via an alternative apoE-dependent pathway. J. Biol. Chem. 270, 28767–28776 (1995).
Nagelkerke, J. F., Havekes, L., van Hinsbergh, V. W. & Van Berkel, T. J. In vivo catabolism of biologically modified LDL. Arteriosclerosis 4, 256–264 (1984).
Merkel, M. et al. Inactive lipoprotein lipase (LPL) alone increases selective cholesterol ester uptake in vivo, whereas in the presence of active LPL it also increases triglyceride hydrolysis and whole particle lipoprotein uptake. J. Biol. Chem. 277, 7405–7411 (2002).
Acknowledgements
The authors thank A. Laatsch, R. Fischer and A. Bartelt for helpful discussions. K. Cornils, B. Holstermann, M. Warmer, S. Ehret and R. Kongi for excellent technical assistance, A. Kornowski for electron microscopy images and R. Capek for providing QD. O.T.B. is supported by a fellowship from the Studienstiftung des Deutschen Volkes. This work was supported by grants from the Deutsche Forschungsgemeinschaft to J.H., U.B. and A.E. (HE3645/2-2, BE829/10-1 and EY16/9-1).
Author information
Authors and Affiliations
Contributions
O.T.B performed and was involved in all aspects of the experiments. O.T.B, U.B. and J.H. designed the study. H.I., K.P., M.G.K. and G.A. performed MRI measurements. U.I.T., N.C.B., M.S.N., S.S., A.E. and H.W. were responsible for synthesis and characterization of nanocrystals. R.R. and H.H. were responsible for electron microscopy. O.T.B, U.I.T., U.B. and J.H. co-wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Supplementary information
Supplementary Information
Supplementary Information (PDF 1233 kb)
Supplementary Information
Supplementary Movie 1 (AVI 6044 kb)
Rights and permissions
About this article
Cite this article
Bruns, O., Ittrich, H., Peldschus, K. et al. Real-time magnetic resonance imaging and quantification of lipoprotein metabolism in vivo using nanocrystals. Nature Nanotech 4, 193–201 (2009). https://doi.org/10.1038/nnano.2008.405
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2008.405
This article is cited by
-
Multimodal X-ray imaging of nanocontainer-treated macrophages and calcium distribution in the perilacunar bone matrix
Scientific Reports (2020)
-
Artificial local magnetic field inhomogeneity enhances T2 relaxivity
Nature Communications (2017)
-
The FMR Behaviour of Li–Ni Ferrite Prepared by Hydrothermal Method
Journal of Superconductivity and Novel Magnetism (2017)
-
Development of multifunctional nanoparticles towards applications in non-invasive magnetic resonance imaging and axonal tracing
JBIC Journal of Biological Inorganic Chemistry (2017)
-
Next-generation in vivo optical imaging with short-wave infrared quantum dots
Nature Biomedical Engineering (2017)