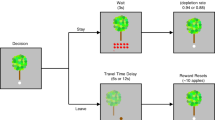
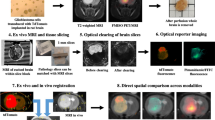
Abstract
Positron emission tomography (PET) neuroimaging and behavioral assays in rodents are widely used in neuroscience. PET gives insights into the molecular processes of neuronal communication, and behavioral methods analyze the actions that are associated with such processes. These methods have not been directly integrated, because PET studies in animals have until now required general anesthesia to immobilize the subject, which precludes behavioral studies. We present a method for imaging awake, behaving rats with PET that allows the simultaneous study of behavior. Key components include the 'rat conscious animal PET' or RatCAP, a miniature portable PET scanner that is mounted on the rat's head, a mobility system that allows considerable freedom of movement, radiotracer administration techniques and methods for quantifying behavior and correlating the two data sets. The simultaneity of the PET and behavioral data provides a multidimensional tool for studying the functions of different brain regions and their molecular constituents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Schultz, W. Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 30, 259–288 (2007).
Clark, J.J. et al. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat. Methods 7, 126–129 (2010).
Westerink, B.H.C. Brain microdialysis and its application for the study of animal behaviour. Behav. Brain Res. 70, 103–124 (1995).
Dombeck, D.A., Khabbaz, A.N., Collman, F., Adelman, T.L. & Tank, D.W. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron 56, 43–57 (2007).
Momosaki, S. et al. Rat-PET study without anesthesia: anesthetics modify the dopamine D1 receptor binding in rat brain. Synapse 54, 207–213 (2004).
Patel, V.D., Lee, D.E., Alexoff, D.L., Dewey, S.L. & Schiffer, W.K. Imaging dopamine release with positron emission tomography (PET) and 11C-raclopride in freely moving animals. Neuroimage 41, 1051–1066 (2008).
Hosoi, R. et al. MicroPET detection of enhanced 18F-FDG utilization by PKA inhibitor in awake rat brain. Brain Res. 1039, 199–202 (2005).
Harada, N., Ohba, H., Fukumoto, D., Kakiuchi, T. & Tsukada, H. Potential of [18F]β-CFT-FE (2β-carbomethoxy-3β-(4-fluorophenyl)-8-(2-[18F]fluoroethyl)nortropane) as a dopamine transporter ligand: a PET study in the conscious monkey brain. Synapse 54, 37–45 (2004).
Itoh, T. et al. PET measurement of the in vivo affinity of 11C-(R)-rolipram and the density of its target, phosphodiesterase-4, in the brains of conscious and anesthetized rats. J. Nucl. Med. 50, 749–756 (2009).
Ferris, C.F. et al. Functional magnetic resonance imaging in conscious animals: a new tool in behavioural neuroscience research. J. Neuroendocrinol. 18, 307–318 (2006).
Kyme, A.Z., Zhou, V.W., Meikle, S.R. & Fulton, R.R. Real-time 3D motion tracking for small animal brain PET. Phys. Med. Biol. 53, 2651–2666 (2008).
Grace, A.A. The tonic/phasic model of dopamine system regulation: its relevance for understanding how stimulant abuse can alter basal ganglia function. Drug Alcohol Depend. 37, 111–129 (1995).
Kravitz, A.V. et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466, 622–626 (2010).
White, N.M. Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction 91, 921–949 (1996).
Wise, R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 5, 483–494 (2004).
Vaska, P. et al. RatCAP: miniaturized head-mounted PET for conscious rodent brain imaging. IEEE Trans. Nucl. Sci. 51, 2718–2722 (2004).
Pratte, J.-F. The RatCAP front-end ASIC. IEEE Trans. Nucl. Sci. 55, 2727–2735 (2008).
Junnarkar, S.S. et al. Next generation of real time data acquisition, calibration and control system for the RatCAP scanner. IEEE Trans. Nucl. Sci. 55, 220–224 (2008).
Park, S.J. et al. Digital coincidence processing for the RatCAP conscious rat brain PET scanner. IEEE Trans. Nucl. Sci. 55, 510–515 (2008).
Innis, R.B. et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J. Cereb. Blood Flow Metab. 27, 1533–1539 (2007).
Laruelle, M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J. Cereb. Blood Flow Metab. 20, 423–451 (2000).
Rabiner, E.A. Imaging of striatal dopamine release elicited with NMDA antagonists: is there anything to be seen? J. Psychopharmacol. 21, 253–258 (2007).
Hassoun, W. et al. PET study of the [11C]raclopride binding in the striatum of the awake cat: effects of anaesthetics and role of cerebral blood flow. Eur. J. Nucl. Med. 30, 141–148 (2003).
Eilam, D. & Szechtman, H. Biphasic effect of D-2 agonist quinpirole on locomotion and movements. Eur. J. Pharmacol. 161, 151–157 (1989).
Li, S.-M. Yawning and locomotor behavior induced by dopamine receptor agonists in mice and rats. Behav. Pharmacol. 21, 171–181 (2010).
Carson, R.E. PET physiological measurements using constant infusion. Nucl. Med. Biol. 27, 657–660 (2000).
Hillegaart, V. & Ahlenius, S. Effects of raclopride on exploratory locomotor activity, treadmill locomotion, conditioned avoidance behaviour and catalepsy in rats: behavioural profile comparisons between raclopride, haloperidol and preclamol. Pharmacol. Toxicol. 60, 350–354 (1987).
van den Boss, R., Cools, A.R. & Ogren, S.-O. Differential effects of the selective D2-antagonist raclopride in the nucleus accumbens of the rat on spontaneous and d-amphetamine–induced activity. Psychopharmacology (Berl.) 95, 447–451 (1988).
Larobina, M., Brunetti, A. & Salvatore, M. Small animal PET: a review of commercially available imaging systems. Curr. Med. Imaging Rev. 2, 187–192 (2006).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates 6th edn., 243 (Elsevier, London, 2007).
Henn, F.A. & Vollmayr, B. Stress models of depression: Forming genetically vulnerable strains. Neurosci. Biobehav. Rev. 29, 799–804 (2005).
Schulz, D., Mirrione, M.M. & Henn, F.A. Cognitive aspects of congenital learned helplessness and its reversal by the monoamine oxidase (MAO)-B inhibitor deprenyl. Neurobiol. Learn. Mem. 93, 291–301 (2010).
Ehrin, E. et al. Preparation of 11C-labelled raclopride, a new potent dopamine receptor antagonist: Preliminary PET studies of cerebral dopamine receptors in the monkey. Int. J. Appl. Radiat. Isot. 36, 269–273 (1985).
Sesack, S.R., Aoki, C. & Pickel, V.M. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J. Neurosci. 14, 88–106 (1994).
Acknowledgements
We thank W. Lenz for mechanical design and fabrication; D. Alexoff for assistance with rat handling; S. Park for coincidence-processing methods; W. Schiffer for assistance with data analysis; C. Reiszel for expertise in catheter design; V. Radeka, R. Lecomte and R. Fontaine for contributions to the electronics; J. Logan for advice on kinetic modeling; J. Fowler and the personnel of the Brookhaven National Laboratory PET center and cyclotron for making the radiotracers available for our studies and N. Volkow for proposing the idea of a conscious-animal PET scanner. The research was carried out at Brookhaven National Laboratory under contract number DE-AC02-98CH10886 with the US Department of Energy and funded by the Department of Energy's Office of Biological and Environmental Research.
Author information
Authors and Affiliations
Contributions
D.S. proposed and carried out most of the rat work, acquired and analyzed behavioral data and wrote the paper. S.S. developed quantitative PET data processing and reconstruction software and acquired and analyzed PET data. S.S.J. developed front-end and data acquisition electronics, software and firmware. J.-F.P. developed the front-end microchip. M.L.P. developed data processing and image reconstruction software. S.P.S. assembled and debugged scanner and mechanics. B.R. and S.H.M. acquired rat data and performed data analysis. S.K. constructed scanner components. F.A.H. contributed to the behavioral neuroimaging experiments. P.O. oversaw and conceived key aspects of the electronics. C.L.W. oversaw development of the scanner, especially the front-end detectors and electronics. D.J.S. oversaw development of the scanner and performed rat studies and data analysis. P.V. oversaw development of the scanner, software and mechanics, performed rat studies and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Note (PDF 162 kb)
Supplementary Software
Raw code and input files necessary to process data from the RatCAP scanner into images, including coincidence processing and image reconstruction. (ZIP 199379 kb)
Rights and permissions
About this article
Cite this article
Schulz, D., Southekal, S., Junnarkar, S. et al. Simultaneous assessment of rodent behavior and neurochemistry using a miniature positron emission tomograph. Nat Methods 8, 347–352 (2011). https://doi.org/10.1038/nmeth.1582
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1582
This article is cited by
-
Adaptive modulation of brain hemodynamics across stereotyped running episodes
Nature Communications (2020)
-
Wide and Deep Imaging of Neuronal Activities by a Wearable NeuroImager Reveals Premotor Activity in the Whole Motor Cortex
Scientific Reports (2019)
-
Estimation of and correction for finite motion sampling errors in small animal PET rigid motion correction
Medical & Biological Engineering & Computing (2019)
-
Mouse Social Interaction Test (MoST): a quantitative computer automated analysis of behavior
Journal of Neural Transmission (2017)
-
The effect of isoflurane on 18F-FDG uptake in the rat brain: a fully conscious dynamic PET study using motion compensation
EJNMMI Research (2016)