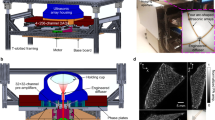
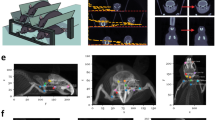
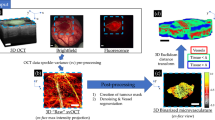
Abstract
Volumetric computed tomography (VCT) is a technology in which area detectors are used for imaging large volumes of a subject with isotropic imaging resolution. We are experimenting with a prototype VCT scanner that uses flat-panel X-ray detectors and is designed for high-resolution three-dimensional (3D) imaging. Using this technique, we have demonstrated microangiography of xeno-transplanted skin squamous cell carcinomas in nude mice. VCT shows the vessel architecture of tumors and animals with greater detail and plasticity than has previously been achieved, and is superior to contrast-enhanced magnetic resonance (MR) angiography. VCT and MR images correlate well for larger tumor vessels, which are tracked from their origin on 3D reconstructions of VCT images. When compared with histology, small tumor vessels with a diameter as small as 50 μm were clearly visualized. Furthermore, imaging small vessel networks inside the tumor tissue improved discrimination of vital and necrotic regions. Thus, VCT substantially improves imaging of vascularization in tumors and offers a promising tool for preclinical studies of tumor angiogenesis and antiangiogenic therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Carmeliet, P. & Jain, R.K. Angiogenesis in cancer and other diseases. Nature 407, 249–257 (2000).
Folkman, J. Angiogenesis-dependent diseases. Semin. Oncol. 28, 536–542 (2001).
Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1, 27–31 (1995).
Fox, S.B., Gasparini, G. & Harris, A.L. Angiogenesis: pathological, prognostic, and growth-factor pathways and their link to trial design and anticancer drugs. Lancet Oncol. 2, 278–289 (2001).
Scappaticci, F.A. Mechanisms and future directions for angiogenesis-based cancer therapies. J. Clin. Oncol. 20, 3906–3927 (2002).
Jain, R.K. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat. Med. 7, 987–989 (2001).
Kerbel, R.S. Tumor angiogenesis: past, present and the near future. Carcinogenesis 21, 505–515 (2000).
Morgan, B. et al. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: results from two phase I studies. J. Clin. Oncol. 21, 3955–3964 (2003).
Port, R.E., Knopp, M.V., Hoffmann, U., Milker-Zabel, S. & Brix, G. Multicompartment analysis of gadolinium chelate kinetics: blood-tissue exchange in mammary tumors as monitored by dynamic MR imaging. J. Magn. Reson. Imaging 10, 233–241 (1999).
Zang, M. & Kono, M. Solitary pulmonary nodules: evaluation of blood flow patterns with dynamic CT. Radiology 205, 471–478 (1997).
Barentsz, J.O. et al. Fast dynamic gadolinium-enhanced MR imaging of urinary bladder and prostate cancer. J. Magn. Reson. Imaging 10, 295–304 (1999).
Fink, C. et al. High-resolution 3D MR angiography of rodent tumors: morphologic characterization of intratumoral vasculature. J. Magn. Reson. Imaging 18, 59–65 (2003).
Kobayashi, H. et al. Micro-MR angiography of normal and intratumoral vessels in mice using dedicated intravascular MR contrast agents with high generation of polyamidoamine dendrimer core: reference to pharmacokinetic properties of dendrimer-based MR contrast agents. J. Magn. Reson. Imaging 14, 705–713 (2001).
Maehara, N. Experimental microcomputed tomography study of the 3D microangioarchitecture of tumors. Eur. Radiol. 13, 1559–1565 (2003).
Lawler, L.P. & Fishman, E.K. Three-dimensional CT angiography with multidetector CT data: study optimization, protocol design, and clinical applications in the abdomen. Crit. Rev. Comput. Tomogr. 43, 77–141 (2002).
Heiken, J.P., Brink J.A. & Vannier M.W. Spiral (helical) CT. Radiology 189, 647–656 (1993).
Wiart, M., Corot, C., Berthezene, Y., Violas, X. & Canet, E. CT pulmonary angiography with a macromolecular contrast medium: a comparative study versus iobitridol in rabbits. Invest. Radiol. 36, 547–553 (2001).
Idee, J.M. et al. Preclinical profile of the monodisperse iodinated macromolecular blood pool agent P743. Invest. Radiol. 36, 41–49 (2001)
Jain, R.K. Molecular regulation of vessel maturation. Nat. Med. 9, 685–693 (2003).
Holash, J. et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 284, 1994–1998 (1999).
Folkman, J. The role of angiogenesis in tumor growth. Semin. Cancer Biol. 3, 65–71 (1992).
Gee, M.S. et al. Tumor vessel development and maturation impose limits on the effectiveness of anti-vascular therapy. Am. J. Pathol. 162, 183–193 (2003).
Jain, R.K. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat. Med. 7, 987–989 (2001).
Fan, X. et al. Differentiation of nonmetastatic and metastatic rodent prostate tumors with high spectral and spatial resolution MRI. Magn. Reson. Med. 45, 1046–1055 (2001).
Miles, K.A., Griffiths, M.R. & Fuentes, M.A. Standardized perfusion value: universal CT contrast enhancement scale that correlates with FDG PET in lung nodules. Radiology 220, 548–553 (2001).
Brix, G. et al. Regional blood flow, capillary permeability, and compartmental volumes: measurement with dynamic CT - initial experience. Radiology 210, 269–276 (1999).
Fusenig, N.E. & Boukamp, P. Multiple stages and genetic alterations in immortalization, malignant transformation, and tumor progression of human skin keratinocytes. J. Mol. Carcinogenesis 23, 144–158 (1998).
Kiessling, F. et al. Dynamic T1 weighted monitoring of vascularization in human carcinoma heterotransplants by magnetic resonance imaging. Int. J. Cancer 104, 113–120 (2003).
Skobe, M., Rockwell, P., Goldstein, N., Vosseler, S. & Fusenig, N.E. Halting angiogenesis suppresses carcinoma cell invasion. Nat. Med. 3, 1222–1227 (1997).
Acknowledgements
We acknowledge colleagues at GE Global Research for developing the VCT research prototype, especially W. Ross, S. Basu and P. FitzGerald for providing algorithm and technical support. Special thanks to P. Edic and P. FitzGerald (GE Global Research) for careful review of the manuscript. Furthermore, we thank B. Misselwitz and J. Meding from Schering AG (Berlin, Germany) for providing us the MR contrast agent and the careful proofreading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kiessling, F., Greschus, S., Lichy, M. et al. Volumetric computed tomography (VCT): a new technology for noninvasive, high-resolution monitoring of tumor angiogenesis. Nat Med 10, 1133–1138 (2004). https://doi.org/10.1038/nm1101
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1101
This article is cited by
-
Predicting a tumour’s drug uptake
Nature Biomedical Engineering (2018)
-
Simultaneous submicrometric 3D imaging of the micro-vascular network and the neuronal system in a mouse spinal cord
Scientific Reports (2015)
-
Ultra High-Resolution In vivo Computed Tomography Imaging of Mouse Cerebrovasculature Using a Long Circulating Blood Pool Contrast Agent
Scientific Reports (2015)
-
Multiscale and multi-modality visualization of angiogenesis in a human breast cancer model
Angiogenesis (2014)
-
Dynamic contrast-enhanced micro-CT on mice with mammary carcinoma for the assessment of antiangiogenic therapy response
European Radiology (2012)