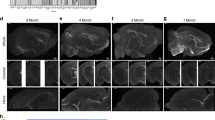
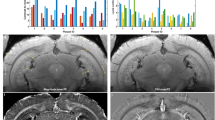
Abstract
The dynamics of β-amyloid deposition and related second-order physiological effects, such as regional cerebral blood flow (rCBF), are key factors for a deeper understanding of Alzheimer's disease (AD). We present longitudinal in vivo data on the dynamics of β-amyloid deposition and the decline of rCBF in two different amyloid precursor protein (APP) transgenic mouse models of AD. Using a multiparametric positron emission tomography and magnetic resonance imaging approach, we demonstrate that in the presence of cerebral β-amyloid angiopathy (CAA), β-amyloid deposition is accompanied by a decline of rCBF. Loss of perfusion correlates with the growth of β-amyloid plaque burden but is not related to the number of CAA-induced microhemorrhages. However, in a mouse model of parenchymal β-amyloidosis and negligible CAA, rCBF is unchanged. Because synaptically driven spontaneous network activity is similar in both transgenic mouse strains, we conclude that the disease-related decline of rCBF is caused by CAA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Weiner, M.W. et al. The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 8, S1–S68 (2012).
Klunk, W.E. et al. Binding of the positron emission tomography tracer Pittsburgh compound-B reflects the amount of amyloid-β in Alzheimer's disease brain but not in transgenic mouse brain. J. Neurosci. 25, 10598–10606 (2005).
Kuntner, C. et al. Limitations of small animal PET imaging with [18F]FDDNP and FDG for quantitative studies in a transgenic mouse model of Alzheimer's disease. Mol. Imaging Biol. 11, 236–240 (2009).
Toyama, H. et al. PET imaging of brain with the β-amyloidprobe, [11C]6-OH-BTA-1, in a transgenic mouse model of Alzheimer's disease. Eur. J. Nucl. Med. Mol. Imaging 32, 593–600 (2005).
Fenchel, M. et al. Perfusion MR imaging with FAIR true FISP spin labeling in patients with and without renal artery stenosis: initial experience. Radiology 238, 1013–1021 (2006).
Raichle, M.E., Herscovitch, P., Mintun, M.A. & Martin, R.W. Cerebral metabolism with positron emission tomography and 0–15 radiopharmaceuticals. Int. J. Neurol. 18, 75–78 (1984).
Snellman, A. et al. Pharmacokinetics of [(1)(8)F]flutemetamol in wild-type rodents and its binding to β-amyloid deposits in a mouse model of Alzheimer's disease. Eur. J. Nucl. Med. Mol. Imaging 39, 1784–1795 (2012).
Rojas, S. et al. In vivo evaluation of amyloid deposition and brain glucose metabolism of 5XFAD mice using positron emission tomography. Neurobiol. Aging 34, 1790–1798 (2013).
Lilja, A.M. et al. Age-dependent neuroplasticity mechanisms in Alzheimer Tg2576 mice following modulation of brain amyloid-β levels. PLoS ONE 8, e58752 (2013).
von Reutern, B. et al. Voxel-based analysis of amyloid-burden measured with [11C]PiB PET in a double transgenic mouse model of Alzheimer's disease. Mol. Imaging Biol. 15, 576–584 (2013).
Snellman, A. et al. Longitudinal amyloid imaging in mouse brain with 11C-PIB: comparison of APP23, Tg2576, and APPswe-PS1dE9 mouse models of Alzheimer disease. J. Nucl. Med. 54, 1434–1441 (2013).
Bateman, R.J. et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N. Engl. J. Med. 367, 795–804 (2012).
Jack, C.R. Jr. et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12, 207–216 (2013).
Jack, C.R. Jr. et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 9, 119–128 (2010).
Villain, N. et al. Regional dynamics of amyloid-β deposition in healthy elderly, mild cognitive impairment and Alzheimer's disease: a voxelwise PiB-PET longitudinal study. Brain 135, 2126–2139 (2012).
Christie, R.H. et al. Growth arrest of individual senile plaques in a model of Alzheimer's disease observed by in vivo multiphoton microscopy. J. Neurosci. 21, 858–864 (2001).
Yan, P. et al. Characterizing the appearance and growth of amyloid plaques in APP/PS1 mice. J. Neurosci. 29, 10706–10714 (2009).
Busche, M.A. et al. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science 321, 1686–1689 (2008).
Radde, R. et al. Aβ42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 7, 940–946 (2006).
Calhoun, M.E. et al. Neuronal overexpression of mutant amyloid precursor protein results in prominent deposition of cerebrovascular amyloid. Proc. Natl. Acad. Sci. USA 96, 14088–14093 (1999).
Maeda, J. et al. Longitudinal, quantitative assessment of amyloid, neuroinflammation, and anti-amyloid treatment in a living mouse model of Alzheimer's disease enabled by positron emission tomography. J. Neurosci. 27, 10957–10968 (2007).
Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat. Rev. Neurosci. 5, 347–360 (2004).
Eichhoff, G., Kovalchuk, Y., Varga, Z., Verkhratsky, A. & Garaschuk, O. Calcium Measurement Methods Vol. 43 (Humana Press, New York, 2010).
Manook, A. et al. Small-animal PET imaging of amyloid-β plaques with [11C]PiB and its multi-modal validation in an APP/PS1 mouse model of Alzheimer's disease. PLoS ONE 7, e31310 (2012).
Wadghiri, Y.Z. et al. Detection of Alzheimer's amyloid in transgenic mice using magnetic resonance microimaging. Magn. Reson. Med. 50, 293–302 (2003).
Yang, J. et al. Detection of amyloid plaques targeted by USPIO-Aβ1–42 in Alzheimer's disease transgenic mice using magnetic resonance microimaging. Neuroimage 55, 1600–1609 (2011).
Hefendehl, J.K. et al. Long-term in vivo imaging of β-amyloid plaque appearance and growth in a mouse model of cerebral β-amyloidosis. J. Neurosci. 31, 624–629 (2011).
Rosen, R.F. et al. Deficient high-affinity binding of Pittsburgh compound B in a case of Alzheimer's disease. Acta Neuropathol. 119, 221–233 (2010).
Bacskai, B.J. et al. Molecular imaging with Pittsburgh compound B confirmed at autopsy: a case report. Arch. Neurol. 64, 431–434 (2007).
Ikonomovic, M.D. et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease. Brain 131, 1630–1645 (2008).
Sabri, O. et al. Multicentre phase 3 trial on florbetaben for β-amyloid brain PET in Alzheimer disease. J. Nucl. Med. Mtg. Abstr. 53, 41 (2012).
Kelly, P.H. et al. Progressive age-related impairment of cognitive behavior in APP23 transgenic mice. Neurobiol. Aging 24, 365–378 (2003).
Wegenast-Braun, B.M. et al. Independent effects of intra- and extracellular Aβ on learning-related gene expression. Am. J. Pathol. 175, 271–282 (2009).
Beckmann, N., Gerard, C., Abramowski, D., Cannet, C. & Staufenbiel, M. Noninvasive magnetic resonance imaging detection of cerebral amyloid angiopathy-related microvascular alterations using superparamagnetic iron oxide particles in APP transgenic mouse models of Alzheimer's disease: application to passive Aβ immunotherapy. J. Neurosci. 31, 1023–1031 (2011).
Winkler, D.T. et al. Spontaneous hemorrhagic stroke in a mouse model of cerebral amyloid angiopathy. J. Neurosci. 21, 1619–1627 (2001).
Phelps, M.E., Hoffman, E.J., Huang, S.C. & Ter-Pogossian, M.M. Effect of positron range on spatial resolution. J. Nucl. Med. 16, 649–652 (1975).
Bao, Q., Newport, D., Chen, M., Stout, D.B. & Chatziioannou, A.F. Performance evaluation of the inveon dedicated PET preclinical tomograph based on the NEMA NU-4 standards. J. Nucl. Med. 50, 401–408 (2009).
Lammertsma, A.A. & Hume, S.P. Simplified reference tissue model for PET receptor studies. Neuroimage 4, 153–158 (1996).
Logan, J. et al. Distribution volume ratios without blood sampling from graphical analysis of PET data. J. Cereb. Blood Flow Metab. 16, 834–840 (1996).
Meyer, E. Simultaneous correction for tracer arrival delay and dispersion in CBF measurements by the H215O autoradiographic method and dynamic PET. J. Nucl. Med. 30, 1069–1078 (1989).
Yee, S.H., Jerabek, P.A. & Fox, P.T. Non-invasive quantification of cerebral blood flow for rats by microPET imaging of 15O labelled water: the application of a cardiac time-activity curve for the tracer arterial input function. Nucl. Med. Commun. 26, 903–911 (2005).
Peller, M. et al. Hyperthermia induces T1 relaxation and blood flow changes in tumors. A MRI thermometry study in vivo. Magn. Reson. Imaging 21, 545–551 (2003).
Zhang, X. et al. In vivo blood T1 measurements at 1.5 T, 3 T, and 7 T. Magn. Reson. Med. 70, 1082–1086 (2013).
Kwong, K.K. et al. MR perfusion studies with T1-weighted echo planar imaging. Magn. Reson. Med. 34, 878–887 (1995).
Davenport, H.A. Histological and Histochemical Technics (W.B. Saunders Company, Philadelphia, USA, 1960).
Sturchler-Pierrat, C. et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease–like pathology. Proc. Natl. Acad. Sci. USA 94, 13287–13292 (1997).
Hsiao, K. et al. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274, 99–102 (1996).
Garaschuk, O., Milos, R.I. & Konnerth, A. Targeted bulk-loading of fluorescent indicators for two-photon brain imaging in vivo. Nat. Protoc. 1, 380–386 (2006).
Stosiek, C., Garaschuk, O., Holthoff, K. & Konnerth, A. In vivo two-photon calcium imaging of neuronal networks. Proc. Natl. Acad. Sci. USA 100, 7319–7324 (2003).
Acknowledgements
This work was funded by the German Federal Ministry of Education and Research (BMBF) grant 01 Gi 0705, the German Research Foundation (DFG) (PI 771/1-1), the Interdisciplinary Center for Clinical Research (IZKF) junior research group program (Functional and Metabolic Brain Imaging, fortüne number 2209-0-0) and the Werner Siemens Foundation. We acknowledge support from P. Martirosian, J. van den Hoff and M. Kneilling. We thank D. Bukala, F. Cay, M. Harant and M. Lehnhoff for their excellent technical assistance during all experiments. We thank J. Odenthal (Department of Cellular Neurology, Hertie-Institute for Clinical Brain Research, University of Tübingen) for providing APPPS1 mice. We thank S.C. Vega for confirmative data evaluation. We thank M. Eichner for statistical expert advice.
Author information
Authors and Affiliations
Contributions
F.C.M. and B.J.P. designed the research. F.C.M., H.F.W., A.M.S., J.G.M., S.W., C.L., L.Y. and O.G. performed the research. M.B., D.S., M.S. and M.J. provided the AD mouse models. A.S. and G.R. provided the PET tracers. F.C.M. and S.W. designed the figures. O.S. provided input of clinical relevance and edited the manuscript. C.C. obtained ethical approval of the study, aided study planning and edited the manuscript. F.C.M. analyzed the data, conducted the statistics and wrote the manuscript. All authors edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
B.J.P. receives grant and research support from AstraZeneca, Bayer Healthcare, Boehringer-Ingelheim, Bruker, Oncodesign, Merck and Siemens; however, none of the grants is directly related to this work. D.S. is an employee of Boehringer-Ingelheim. O.S. receives grant and research support from G.E., Bayer Healthcare, Siemens, Piramal and Navidea; however, none of the grant issuers is directly related to this work. M.S. was an employee of Novartis while the studies were conducted. M.B. is the CEO of Synovo.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Figures 1–9, Supplementary Tables 1–3, Supplementary Discussion and Supplementary Methods. (PDF 2329 kb)
Rights and permissions
About this article
Cite this article
Maier, F., Wehrl, H., Schmid, A. et al. Longitudinal PET-MRI reveals β-amyloid deposition and rCBF dynamics and connects vascular amyloidosis to quantitative loss of perfusion. Nat Med 20, 1485–1492 (2014). https://doi.org/10.1038/nm.3734
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3734
This article is cited by
-
Cerebrovascular Gi Proteins Protect Against Brain Hypoperfusion and Collateral Failure in Cerebral Ischemia
Molecular Imaging and Biology (2023)
-
The brains of aged mice are characterized by altered tissue diffusion properties and cerebral microbleeds
Journal of Translational Medicine (2020)
-
Hybrid PET/MRI enables high-spatial resolution, quantitative imaging of amyloid plaques in an Alzheimer’s disease mouse model
Scientific Reports (2020)
-
Acute targeting of pre-amyloid seeds in transgenic mice reduces Alzheimer-like pathology later in life
Nature Neuroscience (2020)
-
Chemical exchange saturation transfer MRI shows low cerebral 2-deoxy-D-glucose uptake in a model of Alzheimer’s Disease
Scientific Reports (2018)