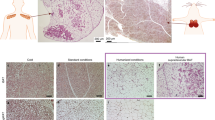
Abstract
The imbalance between energy intake and expenditure is the underlying cause of the current obesity and diabetes pandemics. Central to these pathologies is the fat depot: white adipose tissue (WAT) stores excess calories, and brown adipose tissue (BAT) consumes fuel for thermogenesis using tissue-specific uncoupling protein 1 (UCP1)1,2. BAT was once thought to have a functional role in rodents and human infants only, but it has been recently shown that in response to mild cold exposure, adult human BAT consumes more glucose per gram than any other tissue3. In addition to this nonshivering thermogenesis, human BAT may also combat weight gain by becoming more active in the setting of increased whole-body energy intake4,5,6,7. This phenomenon of BAT-mediated diet-induced thermogenesis has been observed in rodents8 and suggests that activation of human BAT could be used as a safe treatment for obesity and metabolic dysregulation9. In this study, we isolated anatomically defined neck fat from adult human volunteers and compared its gene expression, differentiation capacity and basal oxygen consumption to different mouse adipose depots. Although the properties of human neck fat vary substantially between individuals, some human samples share many similarities with classical, also called constitutive, rodent BAT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004).
Richard, D. & Picard, F. Brown fat biology and thermogenesis. Front. Biosci. 16, 1233–1260 (2011).
Orava, J. et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 14, 272–279 (2011).
Wijers, S.L., Saris, W.H. & Marken Lichtenbelt, W.D. Individual thermogenic responses to mild cold and overfeeding are closely related. J. Clin. Endocrinol. Metab. 92, 4299–4305 (2007).
van Marken Lichtenbelt, W.D. et al. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508 (2009).
Cypess, A.M. et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 (2009).
Saito, M. et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58, 1526–1531 (2009).
Cannon, B. & Nedergaard, J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J. Exp. Biol. 214, 242–253 (2011).
Vernochet, C., McDonald, M.E. & Farmer, S.R. Brown adipose tissue: a promising target to combat obesity. Drug News Perspect. 23, 409–417 (2010).
Zingaretti, M.C. et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 23, 3113–3120 (2009).
Svensson, P.A. et al. Gene expression in human brown adipose tissue. Int. J. Mol. Med. 27, 227–232 (2011).
Cinti, S. Transdifferentiation properties of adipocytes in the adipose organ. Am. J. Physiol. Endocrinol. Metab. 297, E977–E986 (2009).
Morrison, S.F. 2010 Carl Ludwig Distinguished Lectureship of the APS Neural Control and Autonomic Regulation Section: central neural pathways for thermoregulatory cold defense. J. Appl. Physiol. 110, 1137–1149 (2011).
Smith, R.E. Thermoregulatory and adaptive behavior of brown adipose tissue. Science 146, 1686–1689 (1964).
Timmons, J.A. et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc. Natl. Acad. Sci. USA 104, 4401–4406 (2007).
Seale, P. et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967 (2008).
Schulz, T.J. et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc. Natl. Acad. Sci. USA 108, 143–148 (2011).
Petrovic, N. et al. Chronic peroxisome proliferator-activated receptor γ (PPARγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 285, 7153–7164 (2010).
Wu, J. et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012).
Seale, P. et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 6, 38–54 (2007).
Waldén, T.B., Petrovic, N. & Nedergaard, J. PPARα does not suppress muscle-associated gene expression in brown adipocytes but does influence expression of factors that fingerprint the brown adipocyte. Biochem. Biophys. Res. Commun. 397, 146–151 (2010).
Waldén, T.B., Hansen, I.R., Timmons, J.A., Cannon, B. & Nedergaard, J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am. J. Physiol. Endocrinol. Metab. 302, E19–E31 (2012).
Heaton, J.M. The distribution of brown adipose tissue in the human. J. Anat. 112, 35–39 (1972).
Foster, D.O. & Frydman, M.L. Nonshivering thermogenesis in the rat. II. Measurements of blood flow with microspheres point to brown adipose tissue as the dominant site of the calorigenesis induced by noradrenaline. Can. J. Physiol. Pharmacol. 56, 110–122 (1978).
Virtanen, K.A. et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360, 1518–1525 (2009).
Tchkonia, T. et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am. J. Physiol. Endocrinol. Metab. 292, E298–E307 (2007).
Gesta, S., Tseng, Y.H. & Kahn, C.R. Developmental origin of fat: tracking obesity to its source. Cell 131, 242–256 (2007).
Yamamoto, Y. et al. Adipose depots possess unique developmental gene signatures. Obesity (Silver Spring) 18, 872–878 (2010).
Leonardsson, G. et al. Nuclear receptor corepressor RIP140 regulates fat accumulation. Proc. Natl. Acad. Sci. USA 101, 8437–8442 (2004).
Ravussin, E. & Galgani, J.E. The implication of brown adipose tissue for humans. Annu. Rev. Nutr. 31, 33–47 (2011).
Lee, P., Swarbrick, M.M., Zhao, J.T. & Ho, K.K. Inducible brown adipogenesis of supraclavicular fat in adult humans. Endocrinology 152, 3597–3602 (2011).
Tseng, Y.H. et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454, 1000–1004 (2008).
Pisania, A. et al. Quantitative analysis of cell composition and purity of human pancreatic islet preparations. Lab. Invest. 90, 1661–1675 (2010).
Reich, M. et al. GenePattern 2.0. Nat. Genet. 38, 500–501 (2006).
Tchkonia, T. et al. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes 55, 2571–2578 (2006).
Steenhuis, P., Pettway, G.J. & Ignelzi, M.A. Cell surface expression of stem cell antigen-1 (Sca-1) distinguishes osteo-, chondro-, and adipoprogenitors in fetal mouse calvaria. Calcif. Tissue Int. 82, 44–56 (2008).
Kiefer, F.W. et al. Retinaldehyde dehydrogenase 1 regulates a thermogenic program in white adipose tissue. Nat. Med. 18, 918–925 (2012).
Acknowledgements
This work was supported by US National Institutes of Health (NIH) grants DK087317, DK081604, DK046200, DK077097 and P30 DK036836 from the National Institute of Diabetes and Digestive and Kidney Diseases KL2 RR025757 and the Clinical Translational Science Award UL1RR025758 to Harvard University and Beth Israel Deaconess Medical Center from the National Center for Research Resources, Harvard Catalyst, The Harvard Clinical and Translational Science Center (NIH award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers), as well as the Harvard Stem Cell Institute and Eli Lilly Foundation. We thank Y.I. Chen and K.K. Kwong for the magnetic resonance imaging; K. Chaudhary for assistance in figure design; M. Mittleman, H. Keenan and E. Wilker for biostatistical advice; J. Skupien for advice on factor analysis; H. Sacks for insights into the role of brown fat in warming blood; C. Cahill at the Joslin Diabetes Center Microscopy Core for expertise in histology; M. Rourk and G. Smyth for assistance in conducting the rodent studies; the teams of operating room nurses for assistance in tissue collection; and C.R. Kahn for input in preparing this manuscript. Finally, we are grateful to our volunteers for their commitment to the studies.
Author information
Authors and Affiliations
Contributions
A.M.C., A.P.W., R.X. and Y.-H.T. designed the experiments. A.M.C., A.P.W., C.V., T.J.S., C.A.S. and Y.-H.T. wrote the manuscript. T.L.H. did immunohistochemistry. A.M.C., A.P.W., R.X., C.A.S., C.R.-T., L.S.W., C.S., A.T.C., L.N.D., L.M.H., N.T., A.L.G., A.R.H., A.G., P.-O.H., M.A.M. and M.M. did the human gene expression profiling. C.A.S., N.T. and M.A.M. did the mouse gene expression profiling. T.J.S. performed brown adipogenesis experiments. C.V. and C.A.S. performed bioenergenics experiments. All authors contributed to editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.M.C. and Y.-H.T. are recipients of a sponsored research grant from Chugai Pharmaceutical Co., Ltd through Joslin Diabetes Center.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2 and Supplementary Tables 1–6 (PDF 4867 kb)
Rights and permissions
About this article
Cite this article
Cypess, A., White, A., Vernochet, C. et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med 19, 635–639 (2013). https://doi.org/10.1038/nm.3112
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3112
This article is cited by
-
Triiodothyronine (T3) promotes brown fat hyperplasia via thyroid hormone receptor α mediated adipocyte progenitor cell proliferation
Nature Communications (2022)
-
Brown adipose tissue fat-fraction is associated with skeletal muscle adiposity
European Journal of Applied Physiology (2022)
-
Adipogenesis of ear mesenchymal stem cells (EMSCs): adipose biomarker-based assessment of genetic variation, adipocyte function, and brown/brite differentiation
Molecular and Cellular Biochemistry (2022)
-
ADRA1A–Gαq signalling potentiates adipocyte thermogenesis through CKB and TNAP
Nature Metabolism (2022)
-
Regulatory modules of human thermogenic adipocytes: functional genomics of large cohort and Meta-analysis derived marker-genes
BMC Genomics (2021)