Abstract
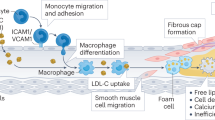
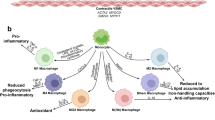
Coronary artery disease (CAD) arising from atherosclerosis is a leading cause of death and morbidity worldwide. The underlying pathogenesis involves an imbalanced lipid metabolism and a maladaptive immune response entailing a chronic inflammation of the arterial wall. The disturbed equilibrium of lipid accumulation, immune responses and their clearance is shaped by leukocyte trafficking and homeostasis governed by chemokines and their receptors. New pro- and anti-inflammatory pathways linking lipid and inflammation biology have been discovered, and genetic profiling studies have unveiled variations involved in human CAD. The growing understanding of the inflammatory processes and mediators has uncovered an intriguing diversity of targetable mechanisms that can be exploited to complement lipid-lowering therapies. Here we aim to systematically survey recently identified molecular mechanisms, translational developments and clinical strategies for targeting lipid-related inflammation in atherosclerosis and CAD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Moore, K.J. & Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 145, 341–355 (2011).
Kwon, G.P., Schroeder, J.L., Amar, M.J., Remaley, A.T. & Balaban, R.S. Contribution of macromolecular structure to the retention of low-density lipoprotein at arterial branch points. Circulation 117, 2919–2927 (2008).
Hansson, G.K. & Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 12, 204–212 (2011).
Ylä-Herttuala, S. et al. Stabilisation of atherosclerotic plaques. Position Paper of the European Society of Cardiology (ESC) Working Group of Atherosclerosis and Vascular Biology. Thromb. Haemost. 106, 1–19 (2011).
Weber, C., Zernecke, A. & Libby, P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat. Rev. Immunol. 8, 802–815 (2008).
Woollard, K.J. & Geissmann, F. Monocytes in atherosclerosis: subsets and functions. Nat. Rev. Cardiol. 7, 77–86 (2010).
Koenen, R.R. & Weber, C. Therapeutic targeting of chemokine interactions in atherosclerosis. Nat. Rev. Drug Discov. 9, 141–153 (2010).
Swirski, F.K. et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 117, 195–205 (2007).
Tacke, F. et al. Monocyte subsets differentially employ CCR2, CCR5 and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117, 185–194 (2007).
Combadière, C. et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6Chi and Ly6Clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 117, 1649–1657 (2008).
Saederup, N., Chan, L., Lira, S.A. & Charo, I.F. Fractalkine deficiency markedly reduces macrophage accumulation and atherosclerotic lesion formation in Ccr2−/− mice: evidence for independent chemokine functions in atherogenesis. Circulation 117, 1642–1648 (2008).
Zernecke, A. & Weber, C. Chemokines in the vascular inflammatory response of atherosclerosis. Cardiovasc. Res. 86, 192–201 (2010).
Veillard, N.R. et al. Differential influence of chemokine receptors CCR2 and CXCR3 in development of atherosclerosis in vivo. Circulation 112, 870–878 (2005).
Heller, E.A. et al. Chemokine CXCL10 promotes atherogenesis by modulating the local balance of effector and regulatory T cells. Circulation 113, 2301–2312 (2006).
van Wanrooij, E.J. et al. CXCR3 antagonist NBI-74330 attenuates atherosclerotic plaque formation in LDL receptor–deficient mice. Arterioscler. Thromb. Vasc. Biol. 28, 251–257 (2008).
Potteaux, S. et al. Role of bone marrow–derived CC-chemokine receptor 5 in the development of atherosclerosis of low-density lipoprotein receptor knockout mice. Arterioscler. Thromb. Vasc. Biol. 26, 1858–1863 (2006).
Braunersreuther, V. et al. Ccr5 but not Ccr1 deficiency reduces development of diet-induced atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 27, 373–379 (2007).
Huo, Y. et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat. Med. 9, 61–67 (2003).
Mause, S.F., von Hundelshausen, P., Zernecke, A., Koenen, R.R. & Weber, C. Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler. Thromb. Vasc. Biol. 25, 1512–1518 (2005).
Veillard, N.R. et al. Antagonism of RANTES receptors reduces atherosclerotic plaque formation in mice. Circ. Res. 94, 253–261 (2004).
Sachais, B.S. et al. Elimination of platelet factor 4 (PF4) from platelets reduces atherosclerosis in C57BL/6 and Apoe−/− mice. Thromb. Haemost. 98, 1108–1113 (2007).
von Hundelshausen, P. et al. Heterophilic interactions of platelet factor 4 and RANTES promote monocyte arrest on endothelium. Blood 105, 924–930 (2005).
Koenen, R.R. et al. Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Nat. Med. 15, 97–103 (2009).
Landsman, L. et al. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood 113, 963–972 (2009).
Weber, C. et al. CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. J. Clin. Invest. 121, 2898–2910 (2011).
Trogan, E. et al. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc. Natl. Acad. Sci. USA 103, 3781–3786 (2006).
Potteaux, S. et al. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J. Clin. Invest. 121, 2025–2036 (2011).
Feig, J.E. et al. LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J. Clin. Invest. 120, 4415–4424 (2010).
Luchtefeld, M. et al. Chemokine receptor 7 knockout attenuates atherosclerotic plaque development. Circulation 122, 1621–1628 (2010).
Bernhagen, J. et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat. Med. 13, 587–596 (2007).
Soehnlein, O. & Lindbom, L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 10, 427–439 (2010).
Curtiss, L.K. & Tobias, P.S. Emerging role of Toll-like receptors in atherosclerosis. J. Lipid Res. 50 (suppl.), S340–S345 (2009).
Seimon, T.A. et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2–dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 12, 467–482 (2010).
Kanellakis, P. et al. High-mobility group box protein 1 neutralization reduces development of diet-induced atherosclerosis in apolipoprotein E–deficient mice. Arterioscler. Thromb. Vasc. Biol. 31, 313–319 (2011).
Zernecke, A. et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ. Res. 102, 209–217 (2008).
Rotzius, P. et al. Distinct infiltration of neutrophils in lesion shoulders in Apoe−/− mice. Am. J. Pathol. 177, 493–500 (2010).
Drechsler, M., Megens, R.T., van Zandvoort, M., Weber, C. & Soehnlein, O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation 122, 1837–1845 (2010).
Yvan-Charvet, L. et al. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via Toll-like receptors and neutrophil infiltration of atherosclerotic lesions. Circulation 118, 1837–1847 (2008).
Soehnlein, O. et al. Neutrophil secretion products pave the way for inflammatory monocytes. Blood 112, 1461–1471 (2008).
Cole, J.E. et al. Unexpected protective role for Toll-like receptor 3 in the arterial wall. Proc. Natl. Acad. Sci. USA 108, 2372–2377 (2011).
Tabas, I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 10, 36–46 (2010).
Papayannopoulos, V. & Zychlinsky, A. NETs: a new strategy for using old weapons. Trends Immunol. 30, 513–521 (2009).
Zhang, Z. et al. Evidence that cathelicidin peptide LL-37 may act as a functional ligand for CXCR2 on human neutrophils. Eur. J. Immunol. 39, 3181–3194 (2009).
Massberg, S. et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 16, 887–896 (2010).
Ludewig, B. et al. Linking immune-mediated arterial inflammation and cholesterol-induced atherosclerosis in a transgenic mouse model. Proc. Natl. Acad. Sci. USA 97, 12752–12757 (2000).
Buono, C. et al. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc. Natl. Acad. Sci. USA 102, 1596–1601 (2005).
Ait-Oufella, H. et al. Measles virus nucleoprotein induces a regulatory immune response and reduces atherosclerosis in mice. Circulation 116, 1707–1713 (2007).
Sasaki, N. et al. Oral anti-CD3 antibody treatment induces regulatory T cells and inhibits the development of atherosclerosis in mice. Circulation 120, 1996–2005 (2009).
Nilsson, J., Hansson, G.K. & Shah, P.K. Immunomodulation of atherosclerosis: implications for vaccine development. Arterioscler. Thromb. Vasc. Biol. 25, 18–28 (2005).
Hermansson, A. et al. Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. J. Exp. Med. 207, 1081–1093 (2010).
Binder, C.J. et al. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J. Clin. Invest. 114, 427–437 (2004).
Tsimikas, S. et al. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J. Lipid Res. 48, 425–433 (2007).
Schiopu, A. et al. Recombinant antibodies to an oxidized low-density lipoprotein epitope induce rapid regression of atherosclerosis in apobec-1−/−/low-density lipoprotein receptor−/− mice. J. Am. Coll. Cardiol. 50, 2313–2318 (2007).
Kyaw, T. et al. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J. Immunol. 185, 4410–4419 (2010).
Ait-Oufella, H. et al. B cell depletion reduces the development of atherosclerosis in mice. J. Exp. Med. 207, 1579–1587 (2010).
Millonig, G. et al. Network of vascular-associated dendritic cells in intima of healthy young individuals. Arterioscler. Thromb. Vasc. Biol. 21, 503–508 (2001).
Jongstra-Bilen, J. et al. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J. Exp. Med. 203, 2073–2083 (2006).
Choi, J.H. et al. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J. Exp. Med. 206, 497–505 (2009).
Galkina, E. et al. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J. Exp. Med. 203, 1273–1282 (2006).
Liu, P. et al. CX3CR1 deficiency impairs dendritic cell accumulation in arterial intima and reduces atherosclerotic burden. Arterioscler. Thromb. Vasc. Biol. 28, 243–250 (2008).
Wu, H. et al. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation 119, 2708–2717 (2009).
Shaposhnik, Z., Wang, X., Weinstein, M., Bennett, B.J. & Lusis, A.J. Granulocyte macrophage colony-stimulating factor regulates dendritic cell content of atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 27, 621–627 (2007).
Zhu, S.N., Chen, M., Jongstra-Bilen, J. & Cybulsky, M.I. GM-CSF regulates intimal cell proliferation in nascent atherosclerotic lesions. J. Exp. Med. 206, 2141–2149 (2009).
Paulson, K.E. et al. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ. Res. 106, 383–390 (2010).
Angeli, V. et al. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity 21, 561–574 (2004).
Gautier, E.L. et al. Conventional dendritic cells at the crossroads between immunity and cholesterol homeostasis in atherosclerosis. Circulation 119, 2367–2375 (2009).
Han, J.W. et al. Vessel wall-embedded dendritic cells induce T cell autoreactivity and initiate vascular inflammation. Circ. Res. 102, 546–553 (2008).
Niessner, A. et al. Synergistic proinflammatory effects of the antiviral cytokine interferon-alpha and Toll-like receptor 4 ligands in the atherosclerotic plaque. Circulation 116, 2043–2052 (2007).
Niessner, A. et al. Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T cell function in the atherosclerotic plaque through interferon-α. Circulation 114, 2482–2489 (2006).
Goossens, P. et al. Myeloid type I interferon signaling promotes atherosclerosis by stimulating macrophage recruitment to lesions. Cell Metab. 12, 142–153 (2010).
Noels, H., Bernhagen, J. & Weber, C. Macrophage migration inhibitory factor: a noncanonical chemokine important in atherosclerosis. Trends Cardiovasc. Med. 19, 76–86 (2009).
Gotsman, I., Sharpe, A.H. & Lichtman, A.H. T cell costimulation and coinhibition in atherosclerosis. Circ. Res. 103, 1220–1231 (2008).
Lievens, D., Eijgelaar, W.J., Biessen, E.A., Daemen, M.J. & Lutgens, E. The multi-functionality of CD40L and its receptor CD40 in atherosclerosis. Thromb. Haemost. 102, 206–214 (2009).
Lievens, D. et al. Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood 116, 4317–4327 (2010).
Lutgens, E. et al. Deficient CD40-TRAF6 signaling in leukocytes prevents atherosclerosis by skewing the immune response toward an antiinflammatory profile. J. Exp. Med. 207, 391–404 (2010).
Greaves, D.R. & Gordon, S. The macrophage scavenger receptor at 30 years of age: current knowledge and future challenges. J. Lipid Res. 50 (suppl.), S282–S286 (2009).
Manning-Tobin, J.J. et al. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler. Thromb. Vasc. Biol. 29, 19–26 (2009).
Stewart, C.R. et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 11, 155–161 (2010).
Thorp, E. et al. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 9, 474–481 (2009).
Hotamisligil, G.S. Endoplasmic reticulum stress and atherosclerosis. Nat. Med. 16, 396–399 (2010).
Erbay, E. et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 15, 1383–1391 (2009).
Yvan-Charvet, L. et al. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J. Clin. Invest. 117, 3900–3908 (2007).
Yvan-Charvet, L. et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 328, 1689–1693 (2010).
Wang, F. et al. Sphingosine-1-phosphate receptor-2 deficiency leads to inhibition of macrophage proinflammatory activities and atherosclerosis in apoE-deficient mice. J. Clin. Invest. 120, 3979–3995 (2010).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Rajamäki, K. et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS ONE 5, e11765 (2010).
Menu, P. et al. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis. 2, e137 (2011).
Epps, K.C. & Wilensky, R.L. Lp-PLA—a novel risk factor for high-risk coronary and carotid artery disease. J. Intern. Med. 269, 94–106 (2011).
Wilensky, R.L. et al. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nat. Med. 14, 1059–1066 (2008).
Liu, J. et al. Circulating platelet-activating factor is primarily cleared by transport, not intravascular hydrolysis by lipoprotein-associated phospholipase A2/ PAF acetylhydrolase. Circ. Res. 108, 469–477 (2011).
Siess, W. et al. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc. Natl. Acad. Sci. USA 96, 6931–6936 (1999).
Zhou, Z. et al. Lipoprotein-derived lysophosphatidic acid promotes atherosclerosis by releasing CXCL1 from the endothelium. Cell Metab. 13, 592–600 (2011).
Teslovich, T.M. et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466, 707–713 (2010).
Schunkert, H., Erdmann, J. & Samani, N.J. Genetics of myocardial infarction: a progress report. Eur. Heart J. 31, 918–925 (2010).
Kathiresan, S. et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat. Genet. 41, 334–341 (2009).
Ripatti, S. et al. A multilocus genetic risk score for coronary heart disease: case-control and prospective cohort analyses. Lancet 376, 1393–1400 (2010).
Schunkert, H. et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 43, 333–338 (2011).
Coronary Artery Disease (C4D) Genetics Consortium. A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat. Genet. 43, 339–344 (2011).
Harismendy, O. et al. 9p21 DNA variants associated with coronary artery disease impair interferon-gamma signalling response. Nature 470, 264–268 (2011).
Mehta, N.N. et al. The novel atherosclerosis locus at 10q11 regulates plasma CXCL12 levels. Eur. Heart J. 32, 963–971 (2011).
Damås, J.K. et al. Stromal cell-derived factor-1alpha in unstable angina: potential antiinflammatory and matrix-stabilizing effects. Circulation 106, 36–42 (2002).
Kiechl, S. et al. Coronary artery disease-related genetic variant on chromosome 10q11 is associated with carotid intima-media thickness and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 30, 2678–2683 (2010).
Zernecke, A. et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci. Signal. 2, ra81 (2009).
Harris, T.A., Yamakuchi, M., Ferlito, M., Mendell, J.T. & Lowenstein, C.J. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc. Natl. Acad. Sci. USA 105, 1516–1521 (2008).
Fichtlscherer, S. et al. Circulating microRNAs in patients with coronary artery disease. Circ. Res. 107, 677–684 (2010).
Wang, Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 (2011).
Andraws, R., Berger, J.S. & Brown, D.L. Effects of antibiotic therapy on outcomes of patients with coronary artery disease: a meta-analysis of randomized controlled trials. J. Am. Med. Assoc. 293, 2641–2647 (2005).
Ray, K.K. & Cannon, C.P. The potential relevance of the multiple lipid-independent (pleiotropic) effects of statins in the management of acute coronary syndromes. J. Am. Coll. Cardiol. 46, 1425–1433 (2005).
Nissen, S.E. Effect of intensive lipid lowering on progression of coronary atherosclerosis: evidence for an early benefit from the Reversal of Atherosclerosis with Aggressive Lipid Lowering (REVERSAL) trial. Am. J. Cardiol. 96, 61F–68F (2005).
Ridker, P.M. et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet 373, 1175–1182 (2009).
Taylor, A.J. et al. Extended-release niacin or ezetimibe and carotid intima-media thickness. N. Engl. J. Med. 361, 2113–2122 (2009).
Lukasova, M., Malaval, C., Gille, A., Kero, J. & Offermanns, S. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J. Clin. Invest. 121, 1163–1173 (2011).
Wallentin, L. et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 361, 1045–1057 (2009).
von Hundelshausen, P. & Weber, C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ. Res. 100, 27–40 (2007).
Sipahi, I. et al. Beta-blockers and progression of coronary atherosclerosis: pooled analysis of 4 intravascular ultrasonography trials. Ann. Intern. Med. 147, 10–18 (2007).
Yusuf, S. et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N. Engl. J. Med. 358, 1547–1559 (2008).
Nissen, S.E. et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. J. Am. Med. Assoc. 290, 2292–2300 (2003).
Navab, M. et al. Structure and function of HDL mimetics. Arterioscler. Thromb. Vasc. Biol. 30, 164–168 (2010).
Serruys, P.W. et al. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation 118, 1172–1182 (2008).
Jandeleit-Dahm, K.A., Calkin, A., Tikellis, C. & Thomas, M. Direct antiatherosclerotic effects of PPAR agonists. Curr. Opin. Lipidol. 20, 24–29 (2009).
Home, P.D. et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 373, 2125–2135 (2009).
Nissen, S.E. & Wolski, K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch. Intern. Med. 170, 1191–1201 (2010).
Lincoff, A.M., Wolski, K., Nicholls, S.J. & Nissen, S.E. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. J. Am. Med. Assoc. 298, 1180–1188 (2007).
Nissen, S.E. et al. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. J. Am. Med. Assoc. 299, 1547–1560 (2008).
Nicholls, S.J., Tuzcu, E.M., Brennan, D.M., Tardif, J.C. & Nissen, S.E. Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: insights from ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation). Circulation 118, 2506–2514 (2008).
Klingenberg, R. & Hansson, G.K. Treating inflammation in atherosclerotic cardiovascular disease: emerging therapies. Eur. Heart J. 30, 2838–2844 (2009).
Weber, C. et al. Structural determinants of MIF functions in CXCR2-mediated inflammatory and atherogenic leukocyte recruitment. Proc. Natl. Acad. Sci. USA 105, 16278–16283 (2008).
Kraemer, S. et al. MIF-chemokine receptor interactions in atherogenesis are dependent on an N-loop-based 2-site binding mechanism. FASEB J. 25, 894–906 (2011).
Gilbert, J. et al. Effect of CC chemokine receptor 2 CCR2 blockade on serum C-reactive protein in individuals at atherosclerotic risk and with a single nucleotide polymorphism of the monocyte chemoattractant protein-1 promoter region. Am. J. Cardiol. 107, 906–911 (2011).
Liehn, E.A. et al. A new monocyte chemotactic protein-1/chemokine CC motif ligand-2 competitor limiting neointima formation and myocardial ischemia/reperfusion injury in mice. J. Am. Coll. Cardiol. 56, 1847–1857 (2010).
Braunersreuther, V. et al. A novel RANTES antagonist prevents progression of established atherosclerotic lesions in mice. Arterioscler. Thromb. Vasc. Biol. 28, 1090–1096 (2008).
Hjerpe, C., Johansson, D., Hermansson, A., Hansson, G.K. & Zhou, X. Dendritic cells pulsed with malondialdehyde modified low density lipoprotein aggravate atherosclerosis in Apoe−/− mice. Atherosclerosis 209, 436–441 (2010).
Habets, K.L. et al. Vaccination using oxidized low-density lipoprotein-pulsed dendritic cells reduces atherosclerosis in LDL receptor-deficient mice. Cardiovasc. Res. 85, 622–630 (2010).
Hermansson, A. et al. Immunotherapy with tolerogenic apolipoprotein B-100–loaded dendritic cells attenuates atherosclerosis in hypercholesterolemic mice. Circulation 123, 1083–1091 (2011).
Klingenberg, R. et al. Intranasal immunization with an apolipoprotein B-100 fusion protein induces antigen-specific regulatory T cells and reduces atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 30, 946–952 (2010).
Acknowledgements
This work was supported by Deutsche Forschungsgemeinschaft (FOR809), the European Research Council and Fondation Leducq. We sincerely apologize to all scientists whose important contributions to the field could not be cited due to space limitations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
C.W. is shareholder of Carolus Therapeutics Inc.
Rights and permissions
About this article
Cite this article
Weber, C., Noels, H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med 17, 1410–1422 (2011). https://doi.org/10.1038/nm.2538
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2538
This article is cited by
-
Copper homeostasis and cuproptosis in atherosclerosis: metabolism, mechanisms and potential therapeutic strategies
Cell Death Discovery (2024)
-
Pelargonidin alleviates acrolein-induced inflammation in human umbilical vein endothelial cells by reducing COX-2 expression through the NF-κB pathway
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Decreased circulating CTRP3 levels in acute and chronic cardiovascular patients
Journal of Molecular Medicine (2024)
-
Ameliorative effect of Stachytarpheta cayennensis extract and vitamins C and E on arsenic, cadmium and lead co-induced toxicity in Wistar rats
Advances in Traditional Medicine (2024)
-
Biomimetic nanoparticles to enhance the reverse cholesterol transport for selectively inhibiting development into foam cell in atherosclerosis
Journal of Nanobiotechnology (2023)