Abstract
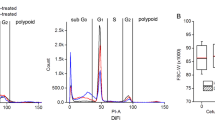
Imaging agents that enable direct visualization and quantification of apoptosis in vivo have great potential value for monitoring chemotherapeutic response as well as for early diagnosis and disease monitoring. We describe here the development of fluorescently labeled activity-based probes (ABPs) that covalently label active caspases in vivo. We used these probes to monitor apoptosis in the thymi of mice treated with dexamethasone as well as in tumor-bearing mice treated with the apoptosis-inducing monoclonal antibody Apomab (Genentech). Caspase ABPs provided direct readouts of the kinetics of apoptosis in live mice, whole organs and tissue extracts. The probes produced a maximum fluorescent signal that could be monitored noninvasively and that coincided with the peak in caspase activity, as measured by gel analysis. Overall, these studies demonstrate that caspase-specific ABPs have the potential to be used for noninvasive imaging of apoptosis in both preclinical and clinical settings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ntziachristos, V. et al. Visualization of antitumor treatment by means of fluorescence molecular tomography with an annexin V–Cy5.5 conjugate. Proc. Natl. Acad. Sci. USA 101, 12294–12299 (2004).
Petrovsky, A., Schellenberger, E., Josephson, L., Weissleder, R. & Bogdanov, A. Jr. Near-infrared fluorescent imaging of tumor apoptosis. Cancer Res. 63, 1936–1942 (2003).
Schellenberger, E.A. et al. Optical imaging of apoptosis as a biomarker of tumor response to chemotherapy. Neoplasia 5, 187–192 (2003).
Blankenberg, F.G., Vanderheyden, J.L., Strauss, H.W. & Tait, J.F. Radiolabeling of HYNIC-annexin V with technetium-99m for in vivo imaging of apoptosis. Nat. Protoc. 1, 108–110 (2006).
Belhocine, T. et al. Increased uptake of the apoptosis-imaging agent (99m)Tc recombinant human Annexin V in human tumors after one course of chemotherapy as a predictor of tumor response and patient prognosis. Clin. Cancer Res. 8, 2766–2774 (2002).
Rottey, S., Slegers, G., Van Belle, S., Goethals, I. & Van de Wiele, C. Sequential 99mTc-hydrazinonicotinamide-annexin V imaging for predicting response to chemotherapy. J. Nucl. Med. 47, 1813–1818 (2006).
Vermeersch, H. et al. 99mTc-HYNIC Annexin-V imaging of primary head and neck carcinoma. Nucl. Med. Commun. 25, 259–263 (2004).
Hofstra, L. et al. Visualisation of cell death in vivo in patients with acute myocardial infarction. Lancet 356, 209–212 (2000).
Aloya, R. et al. Molecular imaging of cell death in vivo by a novel small molecule probe. Apoptosis 11, 2089–2101 (2006).
Cohen, A. et al. From the Gla domain to a novel small-molecule detector of apoptosis. Cell Res. 19, 625–637 (2009).
Laxman, B. et al. Noninvasive real-time imaging of apoptosis. Proc. Natl. Acad. Sci. USA 99, 16551–16555 (2002).
Bullok, K. & Piwnica-Worms, D. Synthesis and characterization of a small, membrane-permeant, caspase-activatable far-red fluorescent peptide for imaging apoptosis. J. Med. Chem. 48, 5404–5407 (2005).
Bauer, C., Bauder-Wuest, U., Mier, W., Haberkorn, U. & Eisenhut, M. 131I-labeled peptides as caspase substrates for apoptosis imaging. J. Nucl. Med. 46, 1066–1074 (2005).
Thornberry, N.A. et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J. Biol. Chem. 272, 17907–17911 (1997).
Kato, D. et al. Activity-based probes that target diverse cysteine protease families. Nat. Chem. Biol. 1, 33–38 (2005).
Sexton, K.B., Witte, M.D., Blum, G. & Bogyo, M. Design of cell-permeable, fluorescent activity–based probes for the lysosomal cysteine protease asparaginyl endopeptidase (AEP)/legumain. Bioorg. Med. Chem. Lett. 17, 649–653 (2007).
Zhou, D. et al. Synthesis, radiolabeling and in vivo evaluation of an 18F-labeled isatin analog for imaging caspase-3 activation in apoptosis. Bioorg. Med. Chem. Lett. 16, 5041–5046 (2006).
Bedner, E., Smolewski, P., Amstad, P. & Darzynkiewicz, Z. Activation of caspases measured in situ by binding of fluorochrome-labeled inhibitors of caspases (FLICA): correlation with DNA fragmentation. Exp. Cell Res. 259, 308–313 (2000).
Smolewski, P. et al. Detection of caspases activation by fluorochrome-labeled inhibitors: multiparameter analysis by laser scanning cytometry. Cytometry 44, 73–82 (2001).
Méthot, N. et al. A caspase active site probe reveals high fractional inhibition needed to block DNA fragmentation. J. Biol. Chem. 279, 27905–27914 (2004).
Pozarowski, P. et al. Interactions of fluorochrome-labeled caspase inhibitors with apoptotic cells: a caution in data interpretation. Cytometry A 55, 50–60 (2003).
Rozman-Pungerčar, J. et al. Inhibition of papain-like cysteine proteases and legumain by caspase-specific inhibitors: when reaction mechanism is more important than specificity. Cell Death Differ. 10, 881–888 (2003).
Berger, A.B. et al. Identification of early intermediates of caspase activation using selective inhibitors and activity-based probes. Mol. Cell 23, 509–521 (2006).
Felsher, D.W. & Bishop, J.M. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol. Cell 4, 199–207 (1999).
Richard, J.P. et al. Cellular uptake of unconjugated TAT peptide involves clathrin-dependent endocytosis and heparan sulfate receptors. J. Biol. Chem. 280, 15300–15306 (2005).
Cohen, J.J. Glucocorticoid-induced apoptosis in the thymus. Semin. Immunol. 4, 363–369 (1992).
Odaka, C. & Mizuochi, T. Macrophages are involved in DNA degradation of apoptotic cells in murine thymus after administration of hydrocortisone. Cell Death Differ. 9, 104–112 (2002).
Brewer, J.A., Kanagawa, O., Sleckman, B.P. & Muglia, L.J. Thymocyte apoptosis induced by T cell activation is mediated by glucocorticoids in vivo. J. Immunol. 169, 1837–1843 (2002).
Zavitsanou, K. et al. Detection of apoptotic cell death in the thymus of dexamethasone treated rats using [123I]annexin V and in situ oligonucleotide ligation. J. Mol. Histol. 38, 313–319 (2007).
Adams, C. et al. Structural and functional analysis of the interaction between the agonistic monoclonal antibody Apomab and the proapoptotic receptor DR5. Cell Death Differ. 15, 751–761 (2008).
Ashkenazi, A. Targeting the extrinsic apoptosis pathway in cancer. Cytokine Growth Factor Rev. 19, 325–331 (2008).
Vivès, E., Brodin, P. & Lebleu, B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 272, 16010–16017 (1997).
Sexton, K.B. et al. Specificity of aza-peptide electrophile activity-based probes of caspases. Cell Death Differ. 14, 727–732 (2007).
Lee, A. & Ellman, J.A. Parallel solution-phase synthesis of mechanism-based cysteine protease inhibitors. Org. Lett. 3, 3707–3709 (2001).
Blum, G., von Degenfeld, G., Merchant, M.J., Blau, H.M. & Bogyo, M. Noninvasive optical imaging of cysteine protease activity using fluorescently quenched activity-based probes. Nat. Chem. Biol. 3, 668–677 (2007).
Acknowledgements
We would like to thank G. Salvesen from the Burnham Institute for Medical Research for the kind gift of recombinant caspases and for creative input on the project. We thank B. Sloane, Wayne State University, for the kind gift of cathepsin antibodies and C. Watts of the University of Dundee for the kind gift of legumain antibodies. We thank R. Weimer for critical discussion of the data and help with protocols for the use of Apomab. We thank A. Fan and D. Felsher for assistance with the MYC mouse model. We thank the Molecular Imaging Program at Stanford and the Stanford Small Animal Imaging Facility for assistance with noninvasive imaging studies. This work was funded by US National Institutes of Health grants U54 RR020843 and R01 EB005011 (to M.B.). M.G.P. was supported by Public Health Services grant CA09302, awarded by the US National Cancer Institute, Department of Health and Human Services.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–7 and Supplementary Methods (PDF 1479 kb)
Rights and permissions
About this article
Cite this article
Edgington, L., Berger, A., Blum, G. et al. Noninvasive optical imaging of apoptosis by caspase-targeted activity-based probes. Nat Med 15, 967–973 (2009). https://doi.org/10.1038/nm.1938
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1938
This article is cited by
-
A fluorogenic-inhibitor-based probe for profiling and imaging of monoamine oxidase A in live human glioma cells and clinical tissues
Science China Chemistry (2023)
-
A self-triggered radioligand therapy agent for fluorescence imaging of the treatment response in prostate cancer
European Journal of Nuclear Medicine and Molecular Imaging (2022)
-
A novel apoptosis probe, cyclic ApoPep-1, for in vivo imaging with multimodal applications in chronic inflammatory arthritis
Apoptosis (2021)
-
In vivo Self-assembled Peptide Nanoprobes for Disease Diagnosis
Chemical Research in Chinese Universities (2021)
-
A fluorogenic cyclic peptide for imaging and quantification of drug-induced apoptosis
Nature Communications (2020)