Abstract
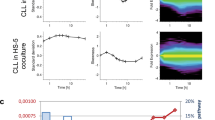
Tissue stromal cells interact with leukaemia cells and profoundly affect their viability and drug sensitivity. Here we show a biochemical mechanism by which bone marrow stromal cells modulate the redox status of chronic lymphocytic leukaemia (CLL) cells and promote cellular survival and drug resistance. Primary CLL cells from patients exhibit a limited ability to transport cystine for glutathione (GSH) synthesis owing to a low expression level of Xc-transporter. In contrast, bone marrow stromal cells effectively import cystine and convert it to cysteine, which is then released into the microenvironment for uptake by CLL cells to promote GSH synthesis. The elevated level of GSH enhances leukaemia cell survival and protects them from drug-induced cytotoxicity. Furthermore, disabling this protective mechanism significantly sensitizes CLL cells to drug treatment in the stromal environment. This stromal–leukaemia interaction is critical for CLL cell survival and represents a key biochemical pathway for effectively targeting leukaemia cells to overcome drug resistance in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Zwiebel, J. A. & Cheson, B. D. Chronic lymphocytic leukemia: staging and prognostic factors. Semin. Oncol. 25, 42–59 (1998).
Keating, M. J. et al. Biology and treatment of chronic lymphocytic leukemia. Hematology/the Education Program of the American Society of Hematology. Am. Soc. Hematol. 153–175 (2003).
Chiorazzi, N., Rai, K. R. & Ferrarini, M. Chronic lymphocytic leukemia. New Engl.J. Med. 352, 804–815 (2005).
Tam, C. S. & Keating, M. J. Chemoimmunotherapy of chronic lymphocytic leukemia. Best Practice Res. 20, 479–498 (2007).
Rai, K. R. & Chiorazzi, N. Determining the clinical course and outcome in chronic lymphocytic leukemia. New Engl. J. Med. 348, 1797–1799 (2003).
Collins, R. J. et al. Spontaneous programmed death (apoptosis) of B-chronic lymphocytic leukaemia cells following their culture in vitro. Br. J. Haematol. 71, 343–350 (1989).
Munk Pedersen, I. & Reed, J. Microenvironmental interactions and survival of CLL B-cells. Leuk. Lymphoma 45, 2365–2372 (2004).
Lagneaux, L., Delforge, A., Bron, D., De Bruyn, C. & Stryckmans, P. Chronic lymphocytic leukemic B cells but not normal B cells are rescued from apoptosis by contact with normal bone marrow stromal cells. Blood 91, 2387–2396 (1998).
Grdisa, M. Influence of CD40 ligation on survival and apoptosis of B-CLL cells in vitro. Leuk. Res. 27, 951–956 (2003).
Dancescu, M. et al. Interleukin 4 protects chronic lymphocytic leukemic B cells from death by apoptosis and upregulates Bcl-2 expression. J. Exp. Med. 176, 1319–1326 (1992).
Jewell, A. P. et al. Interferon- α up-regulates bcl-2 expression and protects B-CLL cells from apoptosis in vitro and in vivo. Br. J. Haematol. 88, 268–274 (1994).
Buschle, M. et al. Interferon γ inhibits apoptotic cell death in B cell chronic lymphocytic leukemia. J. Exp. Med. 177, 213–218 (1993).
Konig, A. et al. Basic fibroblast growth factor (bFGF) upregulates the expression of bcl-2 in B cell chronic lymphocytic leukemia cell lines resulting in delaying apoptosis. Leukemia 11, 258–265 (1997).
Burger, J. A. et al. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood 96, 2655–2663 (2000).
Endo, T. et al. BAFF and APRIL support chronic lymphocytic leukemia B-cellsurvival through activation of the canonical NF-κB pathway. Blood 109, 703–710 (2007).
Hegde, G. V. et al. Hedgehog-induced survival of B-cell chronic lymphocytic leukemia cells in a stromal cell microenvironment: a potential new therapeutic target. Mol. Cancer Res. 6, 1928–1936 (2008).
Lawton, K. A. et al. Analysis of the adult human plasma metabolome. Pharmacogenomics 9, 383–397 (2008).
Sreekumar, A. et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 457, 910–914 (2009).
Dang, L. et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 465, 966 (2010).
Zhou, Y., Hileman, E. O., Plunkett, W., Keating, M. J. & Huang, P. Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents. Blood 101, 4098–4104 (2003).
Oltra, A. M., Carbonell, F., Tormos, C., Iradi, A. & Saez, G. T. Antioxidant enzyme activities and the production of MDA and 8-oxo-dG in chronic lymphocytic leukemia. Free Radic. Biol. Med. 30, 1286–1292 (2001).
Carew, J. S. et al. Mitochondrial DNA mutations in primary leukemia cells after chemotherapy: clinical significance and therapeutic implications. Leukemia 17, 1437–1447 (2003).
Trachootham, D. et al. Effective elimination of fludarabine-resistant CLLcells by PEITC through a redox-mediated mechanism. Blood 112, 1912–1922 (2008).
Silber, R. et al. Glutathione depletion in chronic lymphocytic leukemia B lymphocytes. Blood 80, 2038–2043 (1992).
Hileman, E. O., Liu, J., Albitar, M., Keating, M. J. & Huang, P. Intrinsic oxidative stress in cancer cells: a biochemical basis for therapeutic selectivity. Cancer Chemother. Pharmacol. 53, 209–219 (2004).
Kurtova, A. V. et al. Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance. Blood 114, 4441–4450 (2009).
Woodlock, T. J. et al. Decreased L system amino acid transport and decreased γ-glutamyl transpeptidase are independent processes in human chronic lymphocytic leukemia B-lymphocytes. J. Cell Physiol. 145, 217–221 (1990).
Trachootham, D. et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by β-phenylethyl isothiocyanate. Cancer Cell. 10, 241–252 (2006).
Lu, S. C. Regulation of glutathione synthesis. Curr. Top. Cell. Regul. 36, 95–116 (2000).
Jones, D. P. & Liang, Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic. Biol. Med. 47, 1329–1338 (2009).
Sato, H. et al. Redox imbalance in cystine/glutamate transporter-deficient mice. J. Biol. Chem. 280, 37423–37429 (2005).
Haslinger, C. et al. Microarray gene expression profiling of B-cell chronic lymphocytic leukemia subgroups defined by genomic aberrations and VH mutation status. J. Clin. Oncol. 22, 3937–3949 (2004).
Zenz, T. et al. Detailed analysis of p53 pathway defects in fludarabine-refractory chronic lymphocytic leukemia (CLL): dissecting the contribution of 17p deletion, TP53 mutation, p53-p21 dysfunction, and miR34a in a prospective clinical trial. Blood 114, 2589–2597 (2009).
Turgut, B. et al. 17p Deletion is associated with resistance of B-cell chronic lymphocytic leukemia cells to in vitro fludarabine-induced apoptosis. Leuk. Lymphoma 48, 311–320 (2007).
Huang, Y., Dai, Z., Barbacioru, C. & Sadee, W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance. Cancer Res. 65, 7446–7454 (2005).
Gout, P. W., Buckley, A. R., Simms, C. R. & Bruchovsky, N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia 15, 1633–1640 (2001).
Moufarij, M. A., Sampath, D., Keating, M. J. & Plunkett, W. Fludarabineincreases oxaliplatin cytotoxicity in normal and chronic lymphocytic leukemia lymphocytes by suppressing interstrand DNA crosslink removal. Blood 108, 4187–4193 (2006).
Graham, M. A. et al. Clinical pharmacokinetics of oxaliplatin: a critical review. Clin. Cancer Res. 6, 1205–1218 (2000).
Danhauser, L., Plunkett, W., Keating, M. & Cabanillas, F. 9- β-D-arabinofuranosyl-2-fluoroadenine 5′-monophosphate pharmacokinetics in plasma and tumor cells of patients with relapsed leukemia and lymphoma. Cancer Chemother. Pharmacol. 18, 145–152 (1986).
Bichi, R. et al. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proc. Natl Acad. Sci. USA 99, 6955–6960 (2002).
Reed, J. C. Molecular biology of chronic lymphocytic leukemia. Semin. Oncol. 25, 11–18 (1998).
Caligaris-Cappio, F. Biology of chronic lymphocytic leukemia. Rev. Clin. Exp. Hematol. 4, 5–21 (2000).
Bannai, S. Transport of cystine and cysteine in mammalian cells. Biochim. Biophys. Acta 779, 289–306 (1984).
Iglehart, J. K., York, R. M., Modest, A. P., Lazarus, H. & Livingston, D. M. Cystine requirement of continuous human lymphoid cell lines of normal and leukemic origin. J. Biol. Chem. 252, 7184–7191 (1977).
Uren, J. R. & Lazarus, H. L-cyst(e)ine requirements of malignant cells and progress toward depletion therapy. Cancer Treatment Rep. 63, 1073–1079 (1979).
Hyde, R., Taylor, P. M. & Hundal, H. S. Amino acid transporters: roles in amino acid sensing and signalling in animal cells. Biochem. J. 373, 1–18 (2003).
Liu, R. et al. Cystine-glutamate transporter SLC7A11 mediates resistance to geldanamycin but not to 17-(allylamino)-17-demethoxygeldanamycin. Mol. Pharm. 72, 1637–1646 (2007).
Lo, M., Wang, Y. Z. & Gout, P. W. The x(c)- cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J. Cell. Physiol. 215, 593–602 (2008).
Okuno, S. et al. Role of cystine transport in intracellular glutathione level and cisplatin resistance in human ovarian cancer cell lines. Br. J. Cancer 88, 951–956 (2003).
Chawla, R. K. et al. Plasma cysteine, cystine, and glutathione in cirrhosis. Gastroenterology 87, 770–776 (1984).
Lu, S. C. Regulation of glutathione synthesis. Mol. Aspects Med. 30, 42–59 (2009).
Franco, R., Panayiotidis, M. I. & Cidlowski, J. A. Glutathione depletion is necessary for apoptosis in lymphoid cells independent of reactive oxygen species formation. J. Biol. Chem. 282, 30452–30465 (2007).
Franco, R., DeHaven, W. I., Sifre, M. I., Bortner, C. D. & Cidlowski, J. A. Glutathione depletion and disruption of intracellular ionic homeostasis regulate lymphoid cell apoptosis. J. Biol. Chem. 283, 36071–36087 (2008).
Tew, K. D. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 54, 4313–4320 (1994).
Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. 7, 573–584 (2007).
Roecklein, B. A. & Torok-Storb, B. Functionally distinct human marrow stromal cell lines immortalized by transduction with the human papilloma virus E6/E7 genes. Blood 85, 997–1005 (1995).
Kawano, Y. et al. Ex vivo expansion of human umbilical cord hematopoietic progenitor cells using a coculture system with human telomerase catalytic subunit (hTERT)-transfected human stromal cells. Blood 101, 532–540 (2003).
Matsumoto, S. et al. Membranous osteogenesis system modeled with KUSA-A1 mature osteoblasts. Biochim. Biophys. Acta 1725, 57–63 (2005).
Huang, P., Sandoval, A., Van Den Neste, E., Keating, M. J. & Plunkett, W. Inhibition of RNA transcription: a biochemical mechanism of action against chronic lymphocytic leukemia cells by fludarabine. Leukemia 14, 1405–1413 (2000).
Pelicano, H. et al. Inhibition of mitochondrial respiration: a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J. Biol. Chem. 278, 37832–37839 (2003).
Pepper, C., Thomas, A., Hoy, T., Fegan, C. & Bentley, P. Flavopiridolcircumvents Bcl-2 family mediated inhibition of apoptosis and drugresistance in B-cell chronic lymphocytic leukaemia. Br. J. Haematol. 114, 70–77 (2001).
Campas, C. et al. Prodigiosin induces apoptosis of B and T cells from B-cell chronic lymphocytic leukemia. Leukemia 17, 746–750 (2003).
Acknowledgements
The authors thank D. H. Hawke for expert assistance in LC–MS/MS analysis of cystine and cysteine, and B. A. Hayes, A. G. Melendez, M. A. Ogasawara, L. Feng and H. Zhang for technical assistance and helpful discussions. This work was supported in part by grants CA085563 and CA100428 from the National Institutes of Health, grant PR110322 from CPRIT and a grant for the CLL Global Research Foundation.
Author information
Authors and Affiliations
Contributions
W.Z.: project planning, experimental work, data analysis and manuscript writing; D.T., J.L., G.C., C.G.P. and W.L.: experimental work and data analysis; H.P., J.A.B. and W.P.: project planning and data interpretation; C.M.C.: provided Tcl-1 transgenic mice; M.J.K.: provided CLL specimens and participated in project planning and data interpretation; P.H.: project planning, data analysis and manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1061 kb)
Rights and permissions
About this article
Cite this article
Zhang, W., Trachootham, D., Liu, J. et al. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nat Cell Biol 14, 276–286 (2012). https://doi.org/10.1038/ncb2432
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2432
This article is cited by
-
Targeting ferroptosis in melanoma: cancer therapeutics
Cell Communication and Signaling (2023)
-
Amino acid metabolism in health and disease
Signal Transduction and Targeted Therapy (2023)
-
Targeting metabolic reprogramming in chronic lymphocytic leukemia
Experimental Hematology & Oncology (2022)
-
Induction of apoptosis and autosis in cardiomyocytes by the combination of homocysteine and copper via NOX-mediated p62 expression
Cell Death Discovery (2022)
-
Effect of M2-like macrophages of the injured-kidney cortex on kidney cancer progression
Cell Death Discovery (2022)