Abstract
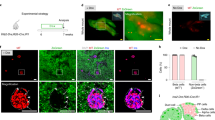
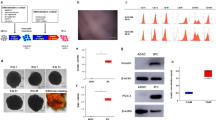
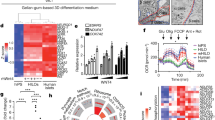
We show that transplantation of adult bone marrow–derived cells expressing c-kit reduces hyperglycemia in mice with streptozotocin-induced pancreatic damage. Although quantitative analysis of the pancreas revealed a low frequency of donor insulin-positive cells, these cells were not present at the onset of blood glucose reduction. Instead, the majority of transplanted cells were localized to ductal and islet structures, and their presence was accompanied by a proliferation of recipient pancreatic cells that resulted in insulin production. The capacity of transplanted bone marrow–derived stem cells to initiate endogenous pancreatic tissue regeneration represents a previously unrecognized means by which these cells can contribute to the restoration of organ function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Weissman, I.L., Sander, M. & German, M.S. Stem cells: units of development, units of regeneration, and units in evolution. Cell 100, 157–168 (2000).
Hattori, K. et al. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1+ stem cells from bone-marrow microenvironment. Nat. Med. 8, 841–849 (2002).
Verfaillie, C.M. Adult stem cells: assessing the case for pluripotency. Trends Cell Biol. 12, 502–508 (2002).
Pittenger, M.F., Mosca, J.D. & McIntosh, K.R. Human mesenchymal stem cells: progenitor cells for cartilage, bone, fat and stroma. Curr. Top. Microbiol. Immunol. 251, 3–11 (2000).
Jiang, Y. et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418, 41–49 (2002).
Jackson, K.A., Mi, T. & Goodell, M.A. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc. Natl. Acad. Sci. USA 96, 14482–14486 (1999).
Lagasse, E. et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 6, 1229–1234 (2000).
Brazelton, T.R., Rossi, F.M., Keshet, G.I. & Blau, H.M. From marrow to brain: expression of neuronal phenotypes in adult mice. Science 290, 1775–1779 (2000).
Krause, D.S. et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 105, 369–377 (2001).
Springer, M.L., Brazelton, T.R., Blau, H.M., Rossi, F.M. & Keshet, G.I. Not the usual suspects: the unexpected sources of tissue regeneration. J. Clin. Invest. 107, 1355–1356 (2001).
Wagers, A.J., Sherwood, R.I., Christensen, J.L. & Weissman, I.L. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 297, 2256–2259 (2002).
Wang, X. et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 422, 897–901 (2003).
Vassilopoulos, G., Wang, P.R. & Russell, D.W. Transplanted bone marrow regenerates liver by cell fusion. Nature 422, 901–904 (2003).
Orlic, D. et al. Bone marrow cells regenerate infarcted myocardium. Nature 410, 701–705 (2001).
Raffii, S. & Lyden, D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat. Med. 9, 702–712 (2003).
Cleaver, O. & Melton, D. Endothelial signaling during development. Nat. Med. 9, 661–668 (2003).
Zysset, T. & Sommer, L. Diabetes alters drug metabolism—in vivo studies in a streptozotozin-diabetic rat model. Experientia 42, 560–562 (1986).
Gerling, I.C., Friedman, H., Greiner, D.L., Shultz, L.D. & Leiter, E.H. Multiple low-dose streptozocin-induced diabetes in NOD-scid/scid mice in the absence of functional lymphocytes. Diabetes 43, 433–440 (1994).
Elliott, J.I., Dewchand, H. & Altmann, D.M. Streptozotocin-induced diabetes in mice lacking αβ T cells. Clin. Exp. Immunol. 109, 116–120 (1997).
Tsirigotis, M. et al. Analysis of ubiquitination in vivo using a transgenic mouse model. Biotechniques 31, 120–126 (2001).
Quesenberry, P. et al. Studies on the regulation of hemopoiesis. Exp. Hematol. 13, 43–48 (1985).
Nakauchi, H., Sudo, K. & Ema, H. Quantitative assessment of the stem cell self-renewal capacity. Ann. N.Y. Acad. Sci. 938, 18–24 (2001).
Ortiz, M. et al. Functional characterization of a novel hematopoietic stem cell and its place in the c-Kit maturation pathway in bone marrow cell development. Immunity 10, 173–182 (1999).
Tsai, R.Y. et al. Plasticity, niches, and the use of stem cells. Dev. Cell 2, 707–712 (2002).
Rajagopal, J., Anderson, W.J., Kume, S., Martinez, O.I. & Melton, D.A. Insulin staining of ES cell progeny from insulin uptake. Science 299, 363 (2003).
Offield, M.F. et al. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 122, 983–995 (1996).
Shih, D.Q. et al. Profound defects in pancreatic β-cell function in mice with combined heterozygous mutations in Pdx-1, Hnf-1α, and Hnf-3β. Proc. Natl. Acad. Sci. USA 99, 3818–3823 (2002).
Sander, M. & German, M.S. The β cell transcription factors and development of the pancreas. J. Mol. Med. 75, 327–340 (1997).
Lammert, E., Cleaver, O. & Melton, D. Induction of pancreatic differentiation by signals from blood vessels. Science 294, 564–567 (2001).
Lammert, E., Cleaver, O. & Melton, D. Role of endothelial cells in early pancreas and liver development. Mech. Dev. 120, 59–64 (2003).
Park, K.I., Teng, Y.D. & Snyder, E.Y. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat. Biotechnol. 20, 1111–1117 (2002).
Ourednik, J., Ourednik, V., Lynch, W.P., Schachner, M. & Snyder, E.Y. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat. Biotechnol. 20, 1103–1110 (2002).
Edlund, H. Pancreatic organogenesis—developmental mechanisms and implications for therapy. Nat. Rev. Genet. 3, 524–532 (2002).
Peters, J., Jurgensen, A. & Kloppel, G. Ontogeny, differentiation and growth of the endocrine pancreas. Virchows Arch. 436, 527–538 (2000).
Zulewski, H. et al. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes 50, 521–533 (2001).
Haluzik, M. & Nedvidkova, J. The role of nitric oxide in the development of streptozotocin-induced diabetes mellitus: experimental and clinical implications. Physiol. Res. 49, S37–42 (2000).
Bhardwaj, G. et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat. Immunol. 2, 172–180 (2001).
Burkart, V. et al. Mice lacking the poly(ADP-ribose) polymerase gene are resistant to pancreatic β-cell destruction and diabetes development induced by streptozocin. Nat. Med. 5, 314–319 (1999).
Acknowledgements
Funding for this research project was provided by the Canadian Institutes of Health Research, Asahi Kasei Corporation, and a fellowship award from the CIHR for D.H., and a Canadian Research Chair in Stem Cell Biology and Regenerative Medicine to M.B. Special thanks to Krysta Levac, Lisheng Wang, Francis Karanu, Julie McBride and Kristin Chadwick for their insights and assistance towards this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Hess, D., Li, L., Martin, M. et al. Bone marrow–derived stem cells initiate pancreatic regeneration. Nat Biotechnol 21, 763–770 (2003). https://doi.org/10.1038/nbt841
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt841
This article is cited by
-
Emerging therapeutic options in the management of diabetes: recent trends, challenges and future directions
International Journal of Obesity (2023)
-
Bone marrow mesenchymal stromal cells for diabetes therapy: touch, fuse, and fix?
Stem Cell Research & Therapy (2022)
-
Reprogramming adipose mesenchymal stem cells into islet β-cells for the treatment of canine diabetes mellitus
Stem Cell Research & Therapy (2022)
-
Circulating Hematopoietic (HSC) and Very-Small Embryonic like (VSEL) Stem Cells in Newly Diagnosed Childhood Diabetes type 1 – Novel Parameters of Beta Cell Destruction/Regeneration Balance and Possible Prognostic Factors of Future Disease Course
Stem Cell Reviews and Reports (2022)
-
Direct lineage tracing reveals Activin-a potential for improved pancreatic homing of bone marrow mesenchymal stem cells and efficient ß-cell regeneration in vivo
Stem Cell Research & Therapy (2020)