Abstract
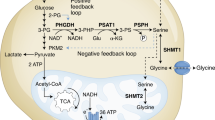
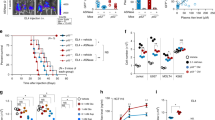
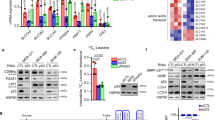
Cancer cells acquire distinct metabolic adaptations to survive stress associated with tumour growth and to satisfy the anabolic demands of proliferation. The tumour suppressor protein p53 (also known as TP53) influences a range of cellular metabolic processes, including glycolysis1,2, oxidative phosphorylation3, glutaminolysis4,5 and anti-oxidant response6. In contrast to its role in promoting apoptosis during DNA-damaging stress, p53 can promote cell survival during metabolic stress7, a function that may contribute not only to tumour suppression but also to non-cancer-associated functions of p538. Here we show that human cancer cells rapidly use exogenous serine and that serine deprivation triggered activation of the serine synthesis pathway and rapidly suppressed aerobic glycolysis, resulting in an increased flux to the tricarboxylic acid cycle. Transient p53-p21 (also known as CDKN1A) activation and cell-cycle arrest promoted cell survival by efficiently channelling depleted serine stores to glutathione synthesis, thus preserving cellular anti-oxidant capacity. Cells lacking p53 failed to complete the response to serine depletion, resulting in oxidative stress, reduced viability and severely impaired proliferation. The role of p53 in supporting cancer cell proliferation under serine starvation was translated to an in vivo model, indicating that serine depletion has a potential role in the treatment of p53-deficient tumours.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bensaad, K. et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 126, 107–120 (2006)
Jiang, P. et al. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nature Cell Biol. 13, 310–120 (2011)
Matoba, S. et al. p53 regulates mitochondrial respiration. Science 312, 1650–1653 (2006)
Suzuki, S. et al. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc. Natl Acad. Sci. USA 107, 7461–7466 (2010)
Hu, W. et al. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc. Natl Acad. Sci. USA 107, 7455–7460 (2010)
Budanov, A. V. et al. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 304, 596–600 (2004)
Jones, R. G. et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell 18, 283–293 (2005)
Maddocks, O. D. K. & Vousden, K. H. Metabolic regulation by p53. J. Mol. Med. 89, 237–245 (2011)
Rose, M. L. et al. Dietary glycine prevents the development of liver tumors caused by the peroxisome proliferator WY-14,643. Carcinogenesis 20, 2075–2081 (1999)
Rose, M. L., Madren, J., Bunzendahl, H. & Thurman, R. G. Dietary glycine inhibits the growth of B16 melanoma tumors in mice. Carcinogenesis 20, 793–798 (1999)
Jain, M. et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science 336, 1040–1044 (2012)
Snell, K. The duality of pathways for serine biosynthesis is a fallacy. Trends Biochem. Sci. 11, 241–243 (1986)
Snell, K. & Fell, D. A. Metabolic control analysis of mammalian serine metabolism. Adv. Enzyme Regul. 30, 13–32 (1990)
Pollari, S. et al. Enhanced serine production by bone metastatic breast cancer cells stimulates osteoclastogenesis. Breast Cancer Res. Treat. 125, 421–430 (2011)
Possemato, R. et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476, 346–350 (2011)
Locasale, J. W. & Cantley, L. C. Genetic selection for enhanced serine metabolism in cancer development. Cell Cycle 10, 3812–3813 (2011)
Ye, J. et al. Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation. Proc. Natl Acad. Sci. USA 109, 6904–6909 (2012)
Kondoh, H. et al. Glycolytic enzymes can modulate cellular life span. Cancer Res. 65, 177–185 (2005)
Mazurek, S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int. J. Biochem. Cell Biol. 43, 969–980 (2011)
Chaneton, B. et al. Serine is a natural ligand and allosteric activator of pyruvate kinase. Nature 491, 458–462 (2012)
Okamura, S. et al. Identification of seven genes regulated by wild-type p53 in a colon cancer cell line carrying a well-controlled wild-type p53 expression system. Oncol. Res. 11, 281–285 (1999)
Stambolsky, P. et al. Regulation of AIF expression by p53. Cell Death Differ. 13, 2140–2149 (2006)
Linke, S. P. et al. A reversible, p53-dependent G0/G1 cell cycle arrest induced by ribonucleotide depletion in the absence of detectable DNA damage. Genes Dev. 10, 934–947 (1996)
Messina, E. et al. Guanine nucleotide depletion triggers cell cycle arrest and apoptosis in human neuroblastoma cell lines. Int. J. Cancer 108, 812–817 (2004)
Deng, C. et al. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82, 675–684 (1995)
Almasan, A. et al. Deficiency of retinoblastoma protein leads to inappropriate S-phase entry, activation of E2F-responsive genes, and apoptosis. Proc. Natl Acad. Sci. USA 92, 5436–5440 (1995)
Dimri, G. P. et al. Inhibition of E2F activity by the cyclin-dependent protein kinase inhibitor p21 in cells expressing or lacking a functional retinoblastoma protein. Mol. Cell. Biol. 16, 2987–2997 (1996)
Grüning, N.-M. & Ralser, M. Cancer: sacrifice for survival. Nature 480, 190–191 (2011)
Anastasiou, D. et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334, 1278–1283 (2011)
Kalhan, S. C. & Hanson, R. W. Resurgence of serine: an often neglected but indispensable amino acid. J. Biol. Chem. 287, 19786–19791 (2012)
Bunz, F. et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282, 1497–1501 (1998)
Donehower, L. A. et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356, 215–221 (1992)
Vigneron, A. M., Ludwig, R. L. & Vousden, K. H. Cytoplasmic ASPP1 inhibits apoptosis through the control of YAP. Genes Dev. 24, 2430–2439 (2010)
Acknowledgements
This work was funded by Cancer Research UK. C.R.B. is a recipient of a Rubicon Fellowship from the Netherlands Organisation for Scientific Research. The authors thank A. Vigneron, B. Chaneton, M. O’Prey, E. Cheung, D. Athineos, G. Kalna, G. Mackay and B. Ludwig for advice and technical assistance.
Author information
Authors and Affiliations
Contributions
K.H.V. and O.D.K.M. conceived the project and wrote the manuscript with C.R.B.’s help. C.R.B. and L.Z. performed and optimized LC–MS, C.R.B. and O.D.K.M. analysed LC–MS raw data. E.G. contributed to the design and interpretation of LC–MS experiments. K.B. and S.M.M. carried out the xenograft experiment, from which K.B. and O.D.K.M. analysed the data. O.D.K.M. performed all other experiments and data analysis. All the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-9. (PDF 1409 kb)
Rights and permissions
About this article
Cite this article
Maddocks, O., Berkers, C., Mason, S. et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 493, 542–546 (2013). https://doi.org/10.1038/nature11743
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11743
This article is cited by
-
Ferroptosis in cancer: From molecular mechanisms to therapeutic strategies
Signal Transduction and Targeted Therapy (2024)
-
Targeting PHGDH reverses the immunosuppressive phenotype of tumor-associated macrophages through α-ketoglutarate and mTORC1 signaling
Cellular & Molecular Immunology (2024)
-
Comprehensive analyses of A 12-metabolism-associated gene signature and its connection with tumor metastases in clear cell renal cell carcinoma
BMC Cancer (2023)
-
Construction of the XGBoost model for early lung cancer prediction based on metabolic indices
BMC Medical Informatics and Decision Making (2023)
-
Tibial fracture surgery in elderly mice caused postoperative neurocognitive disorder via SOX2OT lncRNA in the hippocampus
Molecular Brain (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.