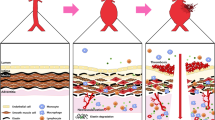
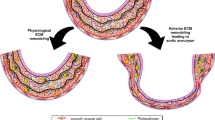
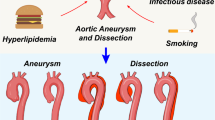
Abstract
Aortic aneurysm is common, accounting for 1–2% of all deaths in industrialized countries. Early theories of the causes of human aneurysm mostly focused on inherited or acquired defects in components of the extracellular matrix in the aorta. Although several mutations in the genes encoding extracellular matrix proteins have been recognized, more recent discoveries have shown important perturbations in cytokine signalling cascades and intracellular components of the smooth muscle contractile apparatus. The modelling of single-gene heritable aneurysm disorders in mice has shown unexpected involvement of the transforming growth factor-β cytokine pathway in aortic aneurysm, highlighting the potential for new therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Szabo, Z. et al. Aortic aneurysmal disease and cutis laxa caused by defects in the elastin gene. J. Med. Genet. 43, 255–258 (2006).
Loeys, B. et al. Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Hum. Mol. Genet. 11, 2113–2118 (2002).
Dasouki, M. et al. Compound heterozygous mutations in fibulin-4 causing neonatal lethal pulmonary artery occlusion, aortic aneurysm, arachnodactyly, and mild cutis laxa. Am. J. Med. Genet. A 143A, 2635–2641 (2007).
Dietz, H. C. TGF-β in the pathogenesis and prevention of disease: a matter of aneurysmic proportions. J. Clin. Invest. 120, 403–407 (2010).
Bergen, A. A. et al. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nature Genet. 25, 228–231 (2000).
Neptune, E. R. et al. Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nature Genet. 33, 407–411 (2003). This study first implicated upregulation of TGF-β activity in the multisystem manifestations of Marfan syndrome.
Habashi, J. P. et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 312, 117–121 (2006).
Loeys, B. L. et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2 . Nature Genet. 37, 275–281 (2005).
Mizuguchi, T. et al. Heterozygous TGFBR2 mutations in Marfan syndrome. Nature Genet. 36, 855–860 (2004).
Loeys, B. L. et al. Aneurysm syndromes caused by mutations in the TGF-β receptor. N. Engl. J. Med. 355, 788–798 (2006).
van de Laar, I. M. B. H. et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nature Genet. 43, 121–126 (2010).
Coucke, P. J. et al. Mutations in the facilitative glucose transporter GLUT10 alter angiogenesis and cause arterial tortuosity syndrome. Nature Genet. 38, 452–457 (2006).
Huang, J. et al. Fibulin-4 deficiency results in ascending aortic aneurysms: a potential link between abnormal smooth muscle cell phenotype and aneurysm progression. Circ. Res. 106, 583–592 (2010).
Horiguchi, M. et al. Fibulin-4 conducts proper elastogenesis via interaction with cross-linking enzyme lysyl oxidase. Proc. Natl Acad. Sci. USA 106, 19029–19034 (2009). This study shows fibulin-4-dependent recruitment of lysyl oxidase to elastin.
Maki, J. M. et al. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation 106, 2503–2509 (2002).
Atsawasuwan, P. et al. Lysyl oxidase binds transforming growth factor-β and regulates its signaling via amine oxidase activity. J. Biol. Chem. 283, 34229–34240 (2008). This biochemical analysis demonstrates direct inactivation of TGF-β ligand by the enzymatic activity of lysyl oxidase.
Zacchigna, L. et al. Emilin1 links TGF-β maturation to blood pressure homeostasis. Cell 124, 929–942 (2006).
Choudhary, B. et al. Absence of TGF-β signaling in embryonic vascular smooth muscle leads to reduced lysyl oxidase expression, impaired elastogenesis, and aneurysm. Genesis 47, 115–121 (2009).
Langlois, D. et al. Conditional inactivation of TGF-β type II receptor in smooth muscle cells and epicardium causes lethal aortic and cardiac defects. Transgenic Res. 19, 1069–1082 (2010).
Arteaga-Solis, E. et al. Regulation of limb patterning by extracellular microfibrils. J. Cell Biol. 154, 275–281 (2001). This study demonstrates that fibrillins can act as positive modulators of TGF-β superfamily function.
Chung, A. W., Yang, H. H., Radomski, M. W. & van Breemen, C. Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in Marfan syndrome through the inhibition of matrix metalloproteinase-2 and -9. Circ. Res. 102, e73–e85 (2008).
Bunton, T. E. et al. Phenotypic alteration of vascular smooth muscle cells precedes elastolysis in a mouse model of Marfan syndrome. Circ. Res. 88, 37–43 (2001).
Sakalihasan, N., Delvenne, P., Nusgens, B. V., Limet, R. & Lapière, C. M. Activated forms of MMP2 and MMP9 in abdominal aortic aneurysms. J. Vasc. Surg. 24, 127–133 (1996).
Moustakas, A. & Heldin, C.-H. The regulation of TGFβ signal transduction. Development 136, 3699–3714 (2009).
Carta, L. et al. p38 MAPK is an early determinant of promiscuous Smad2/3 signaling in the aortas of fibrillin-1 (Fbn1)-null mice. J. Biol. Chem. 284, 5630–5636 (2009).
Holm, T. et al. Noncanonical TGF-β signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science 332, 358–361 (2011).
Habashi, J. P. et al. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science 332, 361–365 (2011).
Yoshimura, K. et al. Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase. Nature Med. 11, 1330–1338 (2005).
Purnell, R., Williams, I., Von Oppell, U. & Wood, A. Giant aneurysms of the sinuses of Valsalva and aortic regurgitation in a patient with Noonan's syndrome. Eur. J. Cardiothorac. Surg. 28, 346–348 (2005).
Friedman, J. M. et al. Cardiovascular disease in neurofibromatosis 1: report of the NF1 Cardiovascular Task Force. Genet. Med. 4, 105–111 (2002).
Tieu, B. C. et al. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J. Clin. Invest. 119, 3637–3651 (2009). This paper describes a major role for adventitial macrophage in aortic dissection in mice.
Onoda, M. et al. Lysyl oxidase resolves inflammation by reducing monocyte chemoattractant protein-1 in abdominal aortic aneurysm. Atherosclerosis 208, 366–369 (2010).
Rodríguez-Vita, J. et al. Angiotensin II activates the Smad pathway in vascular smooth muscle cells by a transforming growth factor-β-independent mechanism. Circulation 111, 2509–2517 (2005).
Wang, Y. et al. TGF-β activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J. Clin. Invest. 120, 422–432 (2010).
King, V. L. et al. Interferon-γ and the interferon-inducible chemokine CXCL10 protect against aneurysm formation and rupture. Circulation 119, 426–435 (2009).
Zhu, L. et al. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nature Genet. 38, 343–349 (2006). This was the first study to implicate the smooth muscle contraction apparatus in thoracic aortic aneurysm.
Pannu, H. et al. MYH11 mutations result in a distinct vascular pathology driven by insulin-like growth factor 1 and angiotensin II. Hum. Mol. Genet. 16, 2453–2462 (2007).
Guo, D.-C. et al. Mutations in smooth muscle α-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nature Genet. 39, 1488–1493 (2007).
Hofmann Bowman, M. et al. S100A12 mediates aortic wall remodeling and aortic aneurysm. Circ. Res. 106, 145–154 (2010).
Sheen, V. L. et al. Filamin A mutations cause periventricular heterotopia with Ehlers–Danlos syndrome. Neurology 64, 254–262 (2005).
Zhu, T.-N. et al. Filamin A-mediated down-regulation of the exchange factor Ras-GRF1 correlates with decreased matrix metalloproteinase-9 expression in human melanoma cells. J. Biol. Chem. 282, 14816–14826 (2007).
Sasaki, A., Masuda, Y., Ohta, Y., Ikeda, K. & Watanabe, K. Filamin associates with Smads and regulates transforming growth factor-β signaling. J. Biol. Chem. 276, 17871–17877 (2001).
Wipff, P.-J., Rifkin, D. B., Meister, J.-J. & Hinz, B. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J. Cell Biol. 179, 1311–1323 (2007).
Hubchak, S. C., Runyan, C. E., Kreisberg, J. I. & Schnaper, H. W. Cytoskeletal rearrangement and signal transduction in TGF-β1-stimulated mesangial cell collagen accumulation. J. Am. Soc. Nephrol. 14, 1969–1980 (2003).
Samarakoon, R., Higgins, C. E., Higgins, S. P. & Higgins, P. J. Differential requirement for MEK/ERK and SMAD signaling in PAI-1 and CTGF expression in response to microtubule disruption. Cell. Signal. 21, 986–995 (2009).
Brooke, B. S. et al. Angiotensin II blockade and aortic-root dilation in Marfan's syndrome. N. Engl. J. Med. 358, 2787–2795 (2008).
Lacro, R. V. et al. Rationale and design of a randomized clinical trial of β-blocker therapy (atenolol) versus angiotensin II receptor blocker therapy (losartan) in individuals with Marfan syndrome. Am. Heart J. 154, 624–631 (2007).
Lee, M. K. et al. TGF-β activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J. 26, 3957–3967 (2007).
Yamashita, M. et al. TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-β. Mol. Cell 31, 918–924 (2008).
Bachman, K. E. & Park, B. H. Duel nature of TGF-β signaling: tumor suppressor vs. tumor promoter. Curr. Opin. Oncol. 17, 49–54 (2005).
Waldo, K. L. et al. Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Dev. Biol. 281, 78–90 (2005).
Majesky, M. W. Developmental basis of vascular smooth muscle diversity. Arterioscler. Thromb. Vasc. Biol. 27, 1248–1258 (2007).
Topouzis, S. & Majesky, M. W. Smooth muscle lineage diversity in the chick embryo: two types of aortic smooth muscle cell differ in growth and receptor-mediated transcriptional responses to transforming growth factor-β. Dev. Biol. 178, 430–445 (1996). This study notes divergent TGF-β responsiveness in different aortic regions.
Carta, L. et al. Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J. Biol. Chem. 281, 8016–8023 (2006).
Dietz, H. C. et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 352, 337–339 (1991).
Li, D. Y. et al. Elastin is an essential determinant of arterial morphogenesis. Nature 393, 276–280 (1998).
Rahkonen, O. et al. Mice with a deletion in the first intron of the Col1a1 gene develop age-dependent aortic dissection and rupture. Circ. Res. 94, 83–90 (2004).
Malfait, F. et al. Three arginine to cysteine substitutions in the pro-α (I)-collagen chain cause Ehlers–Danlos syndrome with a propensity to arterial rupture in early adulthood. Hum. Mutat. 28, 387–395 (2007).
Schwarze, U. et al. Rare autosomal recessive cardiac valvular form of Ehlers–Danlos syndrome results from mutations in the COL1A2 gene that activate the nonsense-mediated RNA decay pathway. Am. J. Hum. Genet. 74, 917–930 (2004).
Liu, X., Wu, H., Byrne, M., Krane, S. & Jaenisch, R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc. Natl Acad. Sci. USA 94, 1852–1856 (1997).
Superti-Furga, A., Gugler, E., Gitzelmann, R. & Steinmann, B. Ehlers–Danlos syndrome type IV: a multi-exon deletion in one of the two COL3A1 alleles affecting structure, stability, and processing of type III procollagen. J. Biol. Chem. 263, 6226–6232 (1988). This was the first genetic study to link a single-gene disorder to aneurysmal disease.
Plaisier, E. et al. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N. Engl. J. Med. 357, 2687–2695 (2007).
Pöschl, E. et al. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 131, 1619–1628 (2004).
Kashtan, C. E. et al. Aortic abnormalities in males with Alport syndrome. Nephrol. Dial. Transplant. 25, 3554–3560 (2010).
Wenstrup, R. J., Murad, S. & Pinnell, S. R. Ehlers–Danlos syndrome type VI: clinical manifestations of collagen lysyl hydroxylase deficiency. J. Pediatr. 115, 405–409 (1989).
Takaluoma, K. et al. Tissue-specific changes in the hydroxylysine content and cross-links of collagens and alterations in fibril morphology in lysyl hydroxylase 1 knock-out mice. J. Biol. Chem. 282, 6588–6596 (2007).
Salo, A. M. et al. A connective tissue disorder caused by mutations of the lysyl hydroxylase 3 gene. Am. J. Hum. Genet. 83, 495–503 (2008).
Ruotsalainen, H. et al. Glycosylation catalyzed by lysyl hydroxylase 3 is essential for basement membranes. J. Cell Sci. 119, 625–635 (2006).
Larsson, J. et al. Abnormal angiogenesis but intact hematopoietic potential in TGF-β type I receptor-deficient mice. EMBO J. 20, 1663–1673 (2001).
Oshima, M., Oshima, H. & Taketo, M. M. TGF-β receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev. Biol. 179, 297–302 (1996).
Hsi, D. H., Ryan, G. F., Hellems, S. O., Cheeran, D. C. & Sheils, L. A. Large aneurysms of the ascending aorta and major coronary arteries in a patient with hereditary hemorrhagic telangiectasia. Mayo Clin. Proc. 78, 774–776 (2003).
Arthur, H. M. et al. Endoglin, an ancillary TGFβ receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev. Biol. 217, 42–53 (2000).
Andersen, N. D. et al. Thoracic endografting in a patient with hereditary hemorrhagic telangiectasia presenting with a descending thoracic aneurysm. J. Vasc. Surg. 51, 468–470 (2010).
Oh, S. P. et al. Activin receptor-like kinase 1 modulates transforming growth factor-β1 signaling in the regulation of angiogenesis. Proc. Natl Acad. Sci. USA 97, 2626–2631 (2000).
Cheng, C.-H. et al. Mutations in the SLC2A10 gene cause arterial abnormalities in mice. Cardiovasc. Res. 81, 381–388 (2009).
Garg, V. et al. Mutations in NOTCH1 cause aortic valve disease. Nature 437, 270–274 (2005).
Conlon, R. A., Reaume, A. G. & Rossant, J. Notch1 is required for the coordinate segmentation of somites. Development 121, 1533–1545 (1995).
Xue, Y. et al. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum. Mol. Genet. 8, 723–730 (1999).
Kamath, B. M. et al. Vascular anomalies in Alagille syndrome: a significant cause of morbidity and mortality. Circulation 109, 1354–1358 (2004).
Distefano, G. et al. Polycystin-1 regulates extracellular signal-regulated kinase-dependent phosphorylation of tuberin to control cell size through mTOR and its downstream effectors S6K and 4EBP1. Mol. Cell. Biol. 29, 2359–2371 (2009).
Hassane, S. et al. Pathogenic sequence for dissecting aneurysm formation in a hypomorphic polycystic kidney disease 1 mouse model. Arterioscler. Thromb. Vasc. Biol. 27, 2177–2183 (2007).
Wu, G. et al. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell 93, 177–188 (1998).
Yu, Q. et al. ENU induced mutations causing congenital cardiovascular anomalies. Development 131, 6211–6223 (2004).
Schildmeyer, L. A. et al. Impaired vascular contractility and blood pressure homeostasis in the smooth muscle α-actin null mouse. FASEB J. 14, 2213–2220 (2000).
Morano, I. et al. Smooth-muscle contraction without smooth-muscle myosin. Nature Cell Biol. 2, 371–375 (2000).
Feng, Y. et al. Filamin A (FLNA) is required for cell–cell contact in vascular development and cardiac morphogenesis. Proc. Natl Acad. Sci. USA 103, 19836–19841 (2006).
Araki, T. et al. Mouse model of Noonan syndrome reveals cell type- and gene dosage-dependent effects of Ptpn11 mutation. Nature Med. 10, 849–857 (2004).
Iwasaki, Y. et al. Coronary artery dilatation in LEOPARD syndrome. A child case and literature review. Congenit. Heart Dis. 4, 38–41 (2009).
Takahashi, H. et al. A hereditary model of slowly progressive polycystic kidney disease in the mouse. J. Am. Soc. Nephrol. 1, 980–989 (1991).
Lee, T. C., Zhao, Y. D., Courtman, D. W. & Stewart, D. J. Abnormal aortic valve development in mice lacking endothelial nitric oxide synthase. Circulation 101, 2345–2348 (2000).
Kuhlencordt, P. J. et al. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation 104, 448–454 (2001).
Cao, J. et al. Thoracic aortic disease in tuberous sclerosis complex: molecular pathogenesis and potential therapies in Tsc2+/− mice. Hum. Mol. Genet. 19, 1908–1920 (2010).
Raben, N. et al. Targeted disruption of the acid α-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. J. Biol. Chem. 273, 19086–19092 (1998).
Rocha, P. P., Scholze, M., Bleiss, W. & Schrewe, H. Med12 is essential for early mouse development and for canonical Wnt and Wnt/PCP signaling. Development 137, 2723–2731 (2010).
Haldar, S. M. et al. Klf15 deficiency is a molecular link between heart failure and aortic aneurysm formation. Sci. Transl. Med. 2, 26ra26 (2010).
Kuo, C. T. et al. The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev. 11, 2996–3006 (1997).
Lopez, L. et al. Turner syndrome is an independent risk factor for aortic dilation in the young. Pediatrics 121, e1622–e1627 (2008).
Daugherty, A., Manning, M. W. & Cassis, L. A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J. Clin. Invest. 105, 1605–1612 (2000). This study formed the basis for the Ang-II-infusion model of aneurysmal disease, the most widespread mouse model of aneurysm.
Anidjar, S. et al. Elastase-induced experimental aneurysms in rats. Circulation 82, 973–981 (1990).
Chiou, A. C., Chiu, B. & Pearce, W. H. Murine aortic aneurysm produced by periarterial application of calcium chloride. J. Surg. Res. 99, 371–376 (2001).
Acknowledgements
We would like to thank E. Arbustini for the use of the multidetector computer tomographic image in Fig. 1a. We acknowledge funding support from the US National Heart, Lung, and Blood Institute, the Howard Hughes Medical Institute, the US National Marfan Foundation and the William S. Smilow Center for Marfan Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reprints and permissions information is available at http://www.nature.com/reprints.
Rights and permissions
About this article
Cite this article
Lindsay, M., Dietz, H. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature 473, 308–316 (2011). https://doi.org/10.1038/nature10145
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10145
This article is cited by
-
Association of MLL3 and TGF-β signaling gene polymorphisms with the susceptibility and prognostic outcomes of Stanford type B aortic dissection
BMC Cardiovascular Disorders (2023)
-
Transforming Growth Factor-β and the Renin-Angiotensin System in Syndromic Thoracic Aortic Aneurysms: Implications for Treatment
Cardiovascular Drugs and Therapy (2021)
-
Inhibition of lysyl oxidase stimulates TGF-β signaling and metalloproteinases-2 and -9 expression and contributes to the disruption of ascending aorta in rats: protection by propylthiouracil
Heart and Vessels (2021)
-
Aortic disease in Marfan syndrome is caused by overactivation of sGC-PRKG signaling by NO
Nature Communications (2021)
-
Quantitative proteomics reveal lineage-specific protein profiles in iPSC-derived Marfan syndrome smooth muscle cells
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.