Abstract
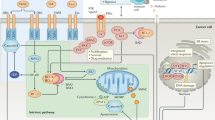
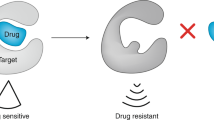
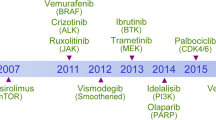
The emergence of tumour-specific, molecularly targeted agents signifies a paradigm shift in cancer therapy, with less reliance on drugs that non-discriminately kill tumour and host cells. Although the diversity of targets giving rise to this new generation of anticancer drugs has expanded, many challenges persist in the design of effective treatment regimens. The complex interplay of signal-transduction pathways further complicates the customization of cancer treatments to target single mechanisms. However, despite uncertainty over precise or dominant mechanisms of action, especially for compounds targeting multiple gene products, emerging agents are producing significant therapeutic advances against a broad range of human cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Johnson, J. I. et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br. J. Cancer 84, 1424–1431 (2001).
Voskoglou-Nomikos, T., Pater, J. L. & Seymour, L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin. Cancer Res. 9, 4227–4239 (2003).
Stehelen, D., Varmus, H. E., Bishop, J. M. & Vogt, P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature 260, 170–173 (1976).
Brugge, J. S. & Erikson, R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature 269, 346–348 (1977).
Collett, M. S. & Erikson, R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc. Natl Acad. Sci. USA 75, 2021–2024 (1978).
Levinson, A. D., Oppermann, H., Levintow, L, Varmus, H. E. & Bishop, J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell 15, 561–572 (1978).
Blume-Jensen, P. & Hunter, T. Oncogenic kinase signalling. Nature 411, 355–365 (2001).
Baselga, J. & Arteaga, C. L. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J. Clin. Oncol. 23, 2445–2459 (2005).
Jain, R. K., Duda, D. G., Clark, J. W. & Loeffler, J. S. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nature Clin. Pract. Oncol. 3, 24–40 (2006).
Gum, R. J. et al. Acquisition of sensitivity of stress-activated protein kinases to the p38 inhibitor, SB 203580, by alteration of one or more amino acids within the ATP binding pocket. J. Biol. Chem. 273, 15605–15610 (1998).
Wang, Z. et al. Structural basis of inhibitor selectivity in MAP kinases. Structure 6, 1117–1128 (1998).
Kostich, M. et al. Human members of the eukaryotic protein kinase family. Genome Biol. 3, RESEARCH0043 (2002).
Manning, G., Whyte, D. B., Martinez, R., Hunter, T. & Sudarsanam, S. The protein kinase complement of the human genome. Science 298, 1912–1934 (2002).
Dudley, D. T, Pang, L., Decker, S. J., Bridges, A. J. & Saltiel, A. R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl Acad. Sci. USA 92, 7686–7689 (1995).
Favata, M. F. et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273, 18623–18632 (1998).
Bain, J., McLauchlan, H., Elliott, M. & Cohen, P. The specificities of protein kinase inhibitors: an update. Biochem. J. 371, 199–204 (2003).
Ohren, J. et al. Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nature Struct. Biol. 11, 1192–1197 (2004).
Schindler, T. et al. Structural mechanism for STI-571 inhibition of Abelson tyrosine kinase. Science 289, 1938–1942 (2000).
Pargellis, C. et al. Inhibition of p38 MAP kinase by utilizing a novel allosteric binding site. Nature Struct. Biol. 9, 268–272 (2002).
Wan, C. et al. Mechanism of activation of the RAF–ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116, 855–867 (2004).
Sheinerman, F. B., Giraud, E. & Laoui, A. High affinity targets of protein kinase inhibitors have similar residues at the positions energetically important for binding. J. Mol. Biol. 352, 1134–1156 (2005).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 7, 57–70 (2000).
Heinrich, M. C., Blanke, C. D., Druker, B. J. & Corless, C. L. Inhibition of KIT tyrosine kinase activity: a novel molecular approach to the treatment of KIT-positive malignancies. J. Clin. Oncol. 20, 1692–1703 (2002).
Shimizu, A. et al. The dermatofibrosarcoma protuberans-associated collagen type Iα1/platelet-derived growth factor (PDGF) B-chain fusion gene generates a transforming protein that is processed to functional PDGF-BB. Cancer Res. 59, 3719–3723 (1999).
Gilliland, D. G. & Griffin, J. D. Role of FLT3 in leukemia. Curr. Opin. Hematol. 9, 274–281 (2002).
Cherrington, J. M., Strawn, L. M. & Shawver, L. K. in Advances in Cancer Research (eds Klein, G., VandeWoude, G. F.) 1–38 (Academic, San Diego, 2000).
Mendel, D. B. et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin. Cancer Res. 9, 327–337 (2003).
Motzer, R. J. et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 24, 16–24 (2006).
Ebos, J. M. et al. A naturally occurring soluble form of vascular endothelial growth factor receptor 2 detected in mouse and human plasma. Mol. Cancer Res. 2, 315–326 (2004).
Lyons, J. F., Wilhelm, S., Hibner, B. & Bollag, G. Discovery of a novel Raf kinase inhibitor. Endocrine Relat. Cancer 8, 219–225 (2001).
Wilhelm, S. & Chien, D.-S. BAY 43-9006: preclinical data. Curr. Pharm. Design 8, 2255–2257 (2002).
Wilhelm, S. M. et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 64, 7099–7109 (2004).
Bergers, G., Song, S., Meyer-Morse, N., Bergsland, E. & Hanahan, D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J. Clin. Invest. 111, 1287–1295 (2003).
Yeon, C. H. & Pegram, M. D. Anti-erbB-2 antibody trastuzumab in the treatment of HER2-amplified breast cancer. Invest. New Drugs 23, 391–409 (2005).
Goldberg, R. M. Cetuximab. Nature Rev. Drug Discov. (suppl. 1), S10–S11 (2005).
Kerr, D. G. Targeting angiogenesis in cancer: clinical development of bevacizumab. Nature Clin. Pract. Oncol. 1, 39–43 (2004).
Cohen, M. H., Williams, G. A., Shridhara, R., Chen, G. & Pazdur, R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist 8, 303–306 (2003).
Johnson, J. R. et al. Approval summary for erlotinib for treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen. Clin. Cancer Res. 11, 6414–6421 (2005).
Nelson, M. H. & Dolder, C. R. Lapatinib: a novel dual tyrosine kinase inhibitor with activity in solid tumors. Ann. Pharmacother. 40, 261–269 (2006).
Fry, D. W. et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol. Cancer Ther. 3, 1427–1438 (2004).
Davies, H. et al. Mutations of the BRAF gene in human cancer. Nature 417, 949–954 (2002).
Satyamoorthy, K. et al. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 63, 756–759 (2003).
Solit, D. B. et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature 439, 358–362 (2006).
Sebolt-Leopold, J. S. & Herrera, R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nature Rev. Cancer 4, 937–947 (2004).
Wallace, E. M., Lyssikatos, J. P., Yeh, T., Winkler, J. D. & Koch, K. Progress towards therapeutic small molecule MEK inhibitors for use in cancer therapy. Curr. Topics Med. Chem. 5, 215–229 (2005).
Gorre, M. C. et al. Clinical resistance to STI-571 cancer therapy caused by BCR–ABL gene mutation or amplification. Science 293, 876–880 (2001).
Tamborini, E. et al. A new mutation in the KIT ATP pocket causes acquired resistance to imatinib in a gastrointestinal stromal tumor patient. Gastroenterology 127, 294–299 (2004).
Kobayashi, S. et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 352, 786–792 (2005).
O'Hare, T., Corbin, A. S. & Druker, B. J. Targeted CML therapy: controlling drug resistance, seeking cure. Curr. Opin. Genet. Dev. 16, 92–99 (2006).
Adrian, F. J. et al. Allosteric inhibitors of Bcr–abl-dependent cell proliferation. Nature Chem. Biol. 2, 95–102 (2006).
Wang, Y. et al. A role for K-ras in conferring resistance to the MEK inhibitor, CI-1040. Neoplasia 7, 336–347 (2005).
Downward, J. Signatures guide drug choice. Nature 439, 274–275 (2006).
She, Q-B. et al. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell 8, 287–297 (2005).
Herbst, R. S. et al. Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J. Clin. Oncol. 23, 2544–2555 (2005).
Hainsworth, J. D. et al. Treatment of metastatic renal cell carcinoma with a combination of bevacizumab and erlotinib. J. Clin. Oncol. 23, 7889–7896 (2005).
Sawyers, C. Targeted cancer therapy. Nature 432, 294–297 (2004).
Canagarajah, B. J., Khokhlatchev, A., Cobb, M. H. & Goldsmith, E. J. Activation mechanism of the MAP kinase ERK2 by dual phosphorylation. Cell 90, 859–869 (1997).
Adams, J. A. Activation loop phosphorylation and catalysis in protein kinases: is there functional evidence for the autoinhibitor model? Biochemistry 42, 601–607 (2003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Both authors are employees of Pfizer Global R&D and own Pfizer stock/shares.
Editor's note: The author has declared interests in Pfizer, which has co-sponsored this Nature Insight. However all editorial content was commissioned entirely independently of this partnership.
Additional information
Author Information Reprints and permissions information is available at npg.nature.com/reprintsandpermissions.
Rights and permissions
About this article
Cite this article
Sebolt-Leopold, J., English, J. Mechanisms of drug inhibition of signalling molecules. Nature 441, 457–462 (2006). https://doi.org/10.1038/nature04874
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04874
This article is cited by
-
Mathematical model of the cell signaling pathway based on the extended Boolean network model with a stochastic process
BMC Bioinformatics (2022)
-
Development of a cell-based pathway modulator screening system to screen the targeted cancer therapeutic candidates
Human Cell (2021)
-
How order and disorder within paramyxoviral nucleoproteins and phosphoproteins orchestrate the molecular interplay of transcription and replication
Cellular and Molecular Life Sciences (2017)
-
Metformin mediated reversal of epithelial to mesenchymal transition is triggered by epigenetic changes in E-cadherin promoter
Journal of Molecular Medicine (2016)
-
RETRACTED ARTICLE: Identification of novel signaling components in N,N’-Dinitrosopiperazine-mediated metastasis of nasopharyngeal Carcinoma by quantitative phosphoproteomics
BMC Cancer (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.