Abstract
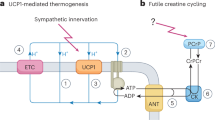
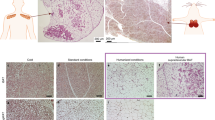
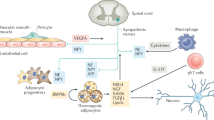
Physiological, pharmacological and genetic studies in dogs, mice and rats have established that the uncoupling protein-1 (UCP1)-based brown adipose tissue system has an important role in the regulation of body temperature. Although it may be possible to create laboratory conditions in which mice with inactivated Ucp1 can survive in a modestly cooled environment, data overwhelmingly support the conclusion that the UCP1/BAT system has evolved to maintain body temperature at 37 °C. The corollary to this conclusion is that any influence UCP1/BAT might have on body weight regulation is a secondary function. The idea that BAT prevents obesity by burning off excess energy to maintain energy balance seems incompatible with evolutionary biology. Premodern humans spent an enormous amount of energy either running to catch their meal or avoiding becoming a meal themselves; consequently, there was no obesity. Nevertheless, although secondary to body temperature regulation, UCP1/BAT is extraordinarily effective at reducing adiposity and insulin resistance in mice and rats. Variation among mice in susceptibility to diet-induced obesity is correlated with the induction of brown adipocytes in traditional white fat depots (wBAT). Both genetic and cell biology-based experimentation have shown that the cellular origins of wBAT are different from those of interscapular-like brown adipocytes (iBAT). Do they have different functions? We have analyzed the effects of the early nutritional environment on the induction of brown adipocytes in inguinal fat to test the hypothesis that wBAT is primarily involved in body weight regulation. Although undernutrition during lactation severely suppresses wBAT at 21 days of age, undernourished mice fed a normal chow diet ad libitum at weaning recovered their normal wBAT and iBAT systems as young adults. The function of wBAT does not seem to be uniquely devoted to body weight regulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009; 360: 1518–1525.
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009; 360: 1500–1508.
Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009; 58: 1526–1531.
Rothwell NJ, Stock MJ . A role for brown adipose tissue in diet-induced thermogenesis. Nature 1979; 281: 31–35.
Trayhurn P, Thurlby PL, James WPT . Thermogenic defect in pre-obese ob/ob mice. Nature 1977; 266: 60–62.
Himms-Hagen J, Desautels M . A mitochondrial defect in brown adipose tissue of the obese (ob/ob) mouse: reduced binding of purine nucleotides and a failure to respond to cold by an increase in binding. Biochem Biophys Res Commun 1978; 83: 628–634.
Prentice AM . Obesity and its potential mechanistic basis. Br Med Bull 2001; 60: 51–67.
Leibel RL, Rosenbaum M, Hirsch J . Changes in energy expenditure resulting from altered body weight. N Engl J Med 1995; 332: 621–628.
Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 1999; 341: 879–884.
Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995; 269: 540–546.
Himms-Hagen J . On raising energy expenditure in ob/ob mice. Science 1997; 276: 1132–1133.
Butler AA, Kozak LP . A recurring problem with analysis of energy expenditure in genetic models of leanness and obesity. Diabetes 2010; 59: 323–329.
Young P, Arch JR, Ashwell M . Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett 1984; 167: 10–14.
Loncar D, Bedrica L, Mayer J, Cannon B, Nedergaard J, Afzelius BA et al. The effect of intermittent cold treatment on the adipose tissue of the cat. Apparent transformation from white to brown adipose tissue. J Ultrastruct Mol Struct Res 1986; 97: 119–129.
Champigny O, Ricquier D, Blondel O, Mayers RM, Briscoe MG, Holloway BR . Beta 3-adrenergic receptor stimulation restores message and expression of brown-fat mitochondrial uncoupling protein in adult dogs. Proc Natl Acad Sci USA 1991; 88: 10774–10777.
Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Penicaud L et al. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci 1992; 103 (Pt 4): 931–942.
Himms-Hagen J, Gorbani M, Claus TH . Reversal of diet-induced obesity (DIO)by CL 316,243, a new thermogenic β3-adrenergic agonist. J Obesity Res 1993; 1: 90S.
Collins S, Daniel KW, Petro AE, Surwit RS . Strain-specific response to beta 3-adrenergic receptor agonist treatment of diet-induced obesity in mice. Endocrinology 1997; 138: 405–413.
Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP . Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest 1998; 102: 412–420.
Coulter AA, Bearden CM, Liu X, Koza RA, Kozak LP . Dietary fat interacts with QTLs controlling induction of Pgc-1 alpha and Ucp1 during conversion of white to brown fat. Physiol Genomics 2003; 14: 139–147.
Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP . Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res 2007; 48: 41–51.
Narvaez CJ, Matthews D, Broun E, Chan M, Welsh J . Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology 2009; 150: 651–661.
Polak P, Cybulski N, Feige JN, Auwerx J, Ruegg MA, Hall MN . Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab 2008; 8: 399–410.
Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008; 454: 961–967.
Berg F, Gustafson U, Andersson L . The uncoupling protein 1 gene (UCP1) is disrupted in the pig lineage: a genetic explanation for poor thermoregulation in piglets. PLoS Genet 2006; 2: e129.
Lean ME, James WP, Jennings G, Trayhurn P . Brown adipose tissue uncoupling protein content in human infants, children and adults. Clin Sci 1986; 71: 291–297.
Koza RA, Hohmann SM, Guerra C, Rossmeisl M, Kozak LP . Synergistic gene interactions control the induction of the mitochondrial uncoupling protein (Ucp1) gene in white fat tissue. J Biol Chem 2000; 275: 34486–34492.
Anunciado-Koza R, Ukropec J, Koza RA, Kozak LP . Inactivation of UCP1 and the glycerol phosphate cycle synergistically increases energy expenditure to resist diet-induced obesity. J Biol Chem 2008; 283: 27688–27697.
Feldmann HM, Golozoubova V, Cannon B, Nedergaard J . UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 2009; 9: 203–209.
Xue B, Coulter A, Rim JS, Koza RA, Kozak LP . Transcriptional synergy and the regulation of Ucp1 during brown adipocyte induction in white fat depots. Mol Cell Biol 2005; 25: 8311–8322.
Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W . Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest 1999; 103: 1489–1498.
Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 2005; 3: e101.
Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK et al. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 2002; 297: 843–845.
Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP . Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest 1995; 96: 2914–2923.
Stefl B, Janovska A, Hodny Z, Rossmeisl M, Horakova M, Syrovy I et al. Brown fat is essential for cold-induced thermogenesis but not for obesity resistance in aP2-Ucp mice. Am J Physiol 1998; 274: E527–E533.
Hamann A, Flier JS, Lowell BB . Decreased brown fat markedly enhances susceptibility to diet-induced obesity, diabetes, and hyperlipidemia. Endocrinology 1996; 137: 21–29.
Acknowledgements
The research described in this paper was supported by the NIH grant R01 HD00841. We thank Tamra Mendoza for outstanding technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
LP Kozak has equity ownership/stock options with Energesis Pharmaceuticals Inc. and has received lecture fees from Novartis. RA Koza has received grant support from Zafgen. R Anunciado-Koza declared no financial interests.
Rights and permissions
About this article
Cite this article
Kozak, L., Koza, R. & Anunciado-Koza, R. Brown fat thermogenesis and body weight regulation in mice: relevance to humans. Int J Obes 34 (Suppl 1), S23–S27 (2010). https://doi.org/10.1038/ijo.2010.179
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2010.179
Keywords
This article is cited by
-
Fat accumulation in striped hamsters (Cricetulus barabensis) reflects the temperature of prior cold acclimation
Frontiers in Zoology (2024)
-
Cold exposure prevents fat accumulation in striped hamsters refed a high-fat diet following food restriction
BMC Zoology (2022)
-
White and brown adipose tissue functionality is impaired by fine particulate matter (PM2.5) exposure
Journal of Molecular Medicine (2022)
-
GHS-R suppression in adipose tissues protects against obesity and insulin resistance by regulating adipose angiogenesis and fibrosis
International Journal of Obesity (2021)
-
Obesity and related consequences to ageing
AGE (2016)