Abstract
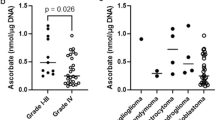
Chromosomal aberrations in human gliomas are principally numerical. In tumours of low malignancy, karyotypes are frequently normal, but occasionally an excess of chromosome 7 and a loss of sex chromosome are observed. In highly malignant tumours, the most frequent aberrations are gain of chromosome 7, loss of chromosome 10 and less frequently losses or deletions of chromosomes 9, 22, 6, 13 and 14 or gains of chromosomes 19 and 20. To understand the meaning of these chromosome imbalances, the relationships between chromosome abnormalities and metabolic disturbances were studied. The losses or deletions observed affected principally chromosomes carrying genes encoding enzymes involved in purine metabolism. The activities of ten enzymes were measured: adenosine kinase, adenine phosphoribosyltransferase, adenylate kinase, methylthioadenosine phosphorylase, hypoxanthine phosphoribosyltransferase, adenylosuccinate lyase, inosine monophosphate dehydrogenase, adenosine deaminase, nucleoside phosphorylase and adenosine monophosphate deaminase. In parallel, two enzymes involved in pyrimidine metabolism, thymidine kinase and thymidylate synthase (TS), were studied. The activities of all these enzymes were measured on samples from 30 human primary glial tumours with low or high malignancy, six xenografted tumours at different passages, four portions of normal brain tissue and four non-glial brain neoplasms. As suggested by cytogenetic data, the enzymatic results showed a relatively low activity of purine metabolism in glial tumours when compared with normal brain and non-glial brain neoplasms. Considering the two enzymes involved in pyrimidine metabolism, only TS had higher activity in glial tumours of high malignancy than in normal brain. In comparison with normal brain, the balance between salvage and de novo pathways changes in gliomas, and even more in grafted tumours, in favour of de novo synthesis. The relation between chromosomes and metabolic imbalances does not correspond to a simple gene dosage effect in these tumours. These data suggest that the decrease of adenosine metabolism occurs before chromosomal aberrations appear, since it is observed in tumours of low malignancy when most karyotypes are still normal, and that the de novo pathway increases with tumour progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bardot, V., Dutrillaux, A., Delattre, J. et al. Purine and pyrimidine metabolism in human gliomas: relation to chromosomal aberrations. Br J Cancer 70, 212–218 (1994). https://doi.org/10.1038/bjc.1994.282
Issue Date:
DOI: https://doi.org/10.1038/bjc.1994.282
This article is cited by
-
Carcinogenic effect of adenylosuccinate lyase (ADSL) in prostate cancer development and progression through the cell cycle pathway
Cancer Cell International (2021)
-
Adenylosuccinate lyase enhances aggressiveness of endometrial cancer by increasing killer cell lectin-like receptor C3 expression by fumarate
Laboratory Investigation (2018)
-
Evaluation of 3′-deoxy-3′-[18F]-fluorothymidine (18F-FLT) kinetics correlated with thymidine kinase-1 expression and cell proliferation in newly diagnosed gliomas
European Journal of Nuclear Medicine and Molecular Imaging (2013)
-
A genome-wide approach identifies that the aspartate metabolism pathway contributes to asparaginase sensitivity
Leukemia (2011)
-
[18F]FLT-PET in oncology: current status and opportunities
European Journal of Nuclear Medicine and Molecular Imaging (2004)