Abstract
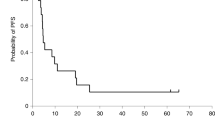
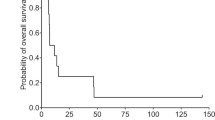
To determine the safety and evaluate the efficacy of repeated administration of virus-producing cells (GLI 328) containing the herpes simplex virus thymidine-kinase gene followed by ganciclovir treatment in adults with recurrent glioblastoma multiforme, we conducted a phase I/II multi-institutional trial. Eligible patients underwent surgical resection of tumor, followed by injections of vector producing cells (VPC) into the brain adjacent to the cavity. An Ommaya reservoir placed after surgery was used to inject a further dose of VPC seven days after surgery, followed seven days later by ganciclovir. Further gene therapy was given at 28-day intervals for up to a total of five cycles. Toxicity and anti-tumor effect were assessed. Of 30 patients who enrolled in the study, 16 experienced serious adverse events possibly related to the experimental therapy. Laboratory testing, including polymerase chain reaction analysis to detect replication-competent retrovirus in peripheral blood lymphocytes and tissues, as well as co-cultivation bioassays, were negative. Before receiving ganciclovir, 37% of the patients showed evidence of transduced peripheral blood leukocytes, but only 12% showed a persistence of transduced cells at the end of the first cycle of ganciclovir. Median survival was 8.4 months. Twenty percent of the patients (n = 6) survived more than 12 months from the date of study entry. This treatment modality is feasible and appears to have some evidence of efficacy. Toxicity may be related in part to the method of gene delivery.
Similar content being viewed by others
References
Fine HA: The basis for current treatment recommendations for malignant gliomas. J Neuro-Oncol 20: 111-120, 1994
Moolten FL: Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res 46: 5276-5281, 1986
Moolten FL, Wells JM: Curability of tumors bearing herpes thymidine kinase genes transferred by retroviral vectors. J Natl Cancer Inst 82: 297-300, 1990
Ezzeddine ZD, Martuza RL, Platika D, Short MP, Malick A, Choi B, Breakefield XO: Selective killing of glioma cells in culture and in vivo by retrovirus transfer of the herpes simplex virus thymidine kinase gene. New Biol 3: 608-614, 1991
Ram Z, Culver KW, Oshiro EM, Viola JJ, DeVroom HL, Otto E, Long Z, Chiang Y, McGarrity GJ, Muul LM, Katz D, Blaese RM, Oldfield EH: Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med 3: 1354-1361, 1997
Klatzmann D, Valery CA, Bensimon G, Marro B, Boyer O, Mokhtari K, Diquet B, Salzmann JL, Philippon J: A phase I/II study of herpes simplex virus type 1 thymidine kinase 'suicide' gene therapy for recurrent glioblastoma. Study Group on Gene Therapy for Glioblastoma. Hum Gene Ther 9: 2595-2604, 1998
Lyons RM, Forry-Schaudies S, Otto E, Wey C, Patil-Koota V, Kaloss M, McGarrity GJ, Chiang YL: An improved retroviral vector encoding the herpes simplex virus thymidine kinase gene increases antitumor efficacy in vivo. Cancer Gene Ther 2: 273-280, 1995
Shand N, Weber F, Mariani L, Bernstein M, Gianella-Borradori A, Long Z, Sorensen AG, Barbier N: A phase 1-2 clinical trial of gene therapy for recurrent glioblastoma multiforme by tumor transduction with the herpes simplex thymidine kinase gene followed by ganciclovir. GLI328 European-Canadian Study Group. Hum Gene Ther 10: 2325-2335, 1999
Packer RJ, Raffel C, Villablanca JG, Tonn JC, Burdach SE, Burger K, LaFond D, McComb JG, Cogen PH, Vezina G, Kapcala LP: Treatment of progressive or recurrent pediatric malignant supratentorial brain tumors with herpes simplex virus thymidine kinase gene vector-producer cells followed by intravenous ganciclovir administration. J Neurosurg 92: 249-254, 2000
Chen CY, Chang YN, Ryan P, Linscott M, McGarrity GJ, Chiang YL: Effect of herpes simplex virus thymidine kinase expression levels on ganciclovir-mediated cytotoxicity and the 'bystander effect'. Hum Gene Ther 6: 1467-1476, 1995
Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blaese RM: In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science 256: 1550-1552, 1992
Smith MM, Thompson JE, Castillo M, Cush S, Mukherji SK, Miller CH, Quattrocchi KB: MR of recurrent high-grade astrocytomas after intralesional immunotherapy. AJNR Am J Neuroradiol 17: 1065-1071, 1996
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Prados, M.D., McDermott, M., Chang, S.M. et al. Treatment of Progressive or Recurrent Glioblastoma Multiforme in Adults with Herpes Simplex Virus Thymidine Kinase Gene Vector-Producer Cells Followed by Intravenous Ganciclovir Administration: A Phase I/II Multi-Institutional Trial. J Neurooncol 65, 269–278 (2003). https://doi.org/10.1023/B:NEON.0000003588.18644.9c
Issue Date:
DOI: https://doi.org/10.1023/B:NEON.0000003588.18644.9c