Abstract
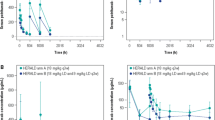
Population pharmacokinetics of sibrotuzumab, a humanized monoclonal antibody directed against fibroblast activation protein, were determined after multiple intravenous infusions of dosages ranging from 5mg/m2 to an absolute dose of 100mg, in patients with advanced or metastatic carcinoma. In total, 1844 serum concentrations from 60 patients in three Phase I and II clinical studies were analyzed. The structural model incorporated two disposition compartments and two parallel elimination pathways from the central compartment, one linear and one nonlinear. Finally estimated pharmacokinetic parameters (%RSE) were: linear clearance CLL 22.1ml/h (9.6), central distribution volume V1 4.13l (3.7), peripheral volume V2 3.19l (8.8), inter-compartmental clearance Q 37.6ml/h (9.6); for the nonlinear clearance Vmax was 0.0338mg/h (25) and Km 0.219μg/ml (57). At serum concentrations between approximately 20ng/ml and 7μg/ml, the effect of the nonlinear clearance on pharmacokinetics was marked. Only at >7μg/ml did CLL dominate overall clearance. Interindividual variability was 57% for CLL, 20% for V1 and V2, and 29% for Vmax and was larger than the inter-occasional variability of 13%. Of the many investigated patient covariates, only body weight was found to contribute to the population model. It significantly affected CLL, V1, V2 and Vmax resulting in marked differences in the model-predicted concentration–time profiles after multiple dosing in patients with low and high body weights. In conclusion, a robust population pharmacokinetic model was developed and evaluated for sibrotuzumab, which identified a possible need to consider body weight when designing dosage regimen for future clinical cancer trials.
Similar content being viewed by others
References
Folkman J, D'Amore PA: Blood vessel formation: What is its molecular basis? Cell 87: 1153–1155, 1996
Hanahan D, Folkman J: Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86: 353–364, 1996
Dvorak HF: Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315: 1650–1659, 1986
Niedermeyer J, Scanlan MJ, Garin-Chesa P, Daiber C, Fiebig HH, Old LJ, Rettig WJ, Schnapp A: Mouse fibroblast activation protein: Molecular cloning, alternative splicing and expression in the reactive stroma of epithelial cancers. Int J Cancer 71: 383–389, 1997
Iozzo RV: Tumor stroma as a regulator of neoplastic behavior. Agonistic and antagonistic elements embedded in the same connective tissue. Lab Invest 73: 157–160, 1995
Garin-Chesa P, Old LJ, Rettig WJ: Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci USA 87: 7235–7239, 1990
Park JE, Lenter MC, Zimmermann RN, Garin-Chesa P, Old LJ, Rettig WJ: Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J Biol Chem 274: 36505–36512, 1999
Cheng JD, Dunbrack RL, Jr, Valianou M, Rogatko A, Alpaugh RK, Weiner LM: Promotion of tumor growth by murine fibroblast activation protein, a serine protease, in an animal model. Cancer Res 62: 4767–4772, 2002
Scanlan MJ, Raj BK, Calvo B, Garin-Chesa P, Sanz-Moncasi MP, Healey JH, Old LJ, Rettig WJ: Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc Natl Acad Sci USA 91: 5657–5661, 1994
Deland FA: A perspective of monoclonal antibodies: Past, present, future. Sem Nucl Med 29: 158–165, 1989
Press MF, Bernstein L, Thomas PA, Meisner LF, Zhou JY, Ma Y, Hung G, Robinson RA, Harris C, El-Naggar A, Slamon DJ, Phillips RN, Ross JS, Wolman SR, Flom KJ: HER-2/neu gene amplification characterized by fluorescence in situ hybridization: Poor prognosis in node-negative breast carcinomas. J Clin Oncol 15: 2894–2904, 1997
Rettig WJ, Su SL, Fortunato SR, Scanlan MJ, Raj BK, Garin-Chesa P, Healey JH, Old LJ: Fibroblast activation protein: Purification, epitope mapping and induction by growth factors. Int J Cancer 58: 385–392, 1994
Welt S, Divgi CR, Scott AM, Garin-Chesa P, Finn RD, Graham M, Carswell EA, Cohen A, Larson SM, Old LJ, Rettig WJ: Antibody targeting in metastatic colon cancer: A phase I study of monoclonal antibody F19 against a cell-surface protein of reactive tumor stromal fibroblasts. J Clin Oncol 12: 1193–1203, 1994
Tanswell P, Garin-Chesa P, Rettig WJ, Welt S, Divgi CR, Casper ES, Finn RD, Larson SM, Old LJ, Scott AM: Population pharmacokinetics of antifibroblast activation protein monoclonal antibody F19 in cancer patients. Br J Clin Pharmacol 51: 177–180, 2001
Scott AW, Wiseman G, Welt S, Lee F-T, Hopkins W, Mitchell P, Adjei A, Divgi C, Larson S, Hoffman E, Tanswell P, Bette P, Amelsberg A, Rettig W: A phase I dose-escalation study of BIBH 1 in patients with advanced or metastatic fibroblast activation protein positive cancer. Proc Am Soc Clin Oncol 20: 258a, 2001
Hofheinz R, Al-Batran SE, Hartmann F, Hartung G, Jäger D, Renner C, Tanswell P, Kunz U, Amelsberg A, Kuthan H, Stehle G: Stromal antigen targeting by a humanised monoclonal antibody: An early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie 26: 44–48, 2003
Ritter G, Cohen LS, Williams C, Jr, Richards EC, Old LJ, Welt S: Serological analysis of human anti-human antibody responses in colon cancer patients treated with repeated doses of humanized monoclonal antibody A33. Cancer Res 61: 6851–6859, 2001
Beal SL, Sheiner LB: NONMEM Users Guide. NONMEM Project Group, University of California San Francisco, CA, 1992
Wahlby U, Jonsson EN, Karlsson MO: Assessment of actual significance levels for covariate effects in NONMEM. J Pharmacokinet Pharmacodyn 28: 231–252, 2001
Jonsson EN, Karlsson MO: Xpose-an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 58: 51–64, 1999
Mandema JW, Verotta D, Sheiner LB: Building population pharmacokinetic-pharmacodynamic models. I. Models for covariate effects. J Pharmacokinet Biopharm 20: 511–528, 1992
Mandema JW, Verotta D, Sheiner LB: Building population pharmacokinetic-pharmacodynamic models. In Advanced Methods of Pharmacokinetic-Pharmacodynamic Systems Analysis. Plenum Press, 1993
Jonsson EN, Karlsson MO: Automated covariate model building within NONMEM. Pharm Res 15: 1463–1468, 1998
Yano Y, Beal SL, Sheiner LB: Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J Pharmacokinet Pharmacodyn 28: 171–192, 2001
Webster WB, Harwood SJ, Carroll RG, Morrissey MA: Pharmacokinetics of indium-111-labeled B72.3 monoclonal antibody in colorectal cancer patients. J Nucl Med 33: 498–504, 1992
Bauer RJ, Dedrick RL, White ML, Murray MJ, Garovoy MR: Population pharmacokinetics and pharmacodynamics of the anti-CD11a antibody hu1124 in human subjects with psoriasis. J Pharmacokinet Biopharm 27: 397–420, 1999
Mentre F, Kovarik J, Gerbeau C: Constructing a prediction interval for time to reach a threshold concentration based on a population pharmacokinetic analysis: An application to basiliximab in renal transplantation. J Pharmacokinet Biopharm 27: 213–230, 1999
Kovarik J, Wolf P, Cisterne JM, Mourad G, Lebranchu Y, Lang P, Bourbigot B, Cantarovich D, Girault D, Gerbeau C, Schmidt AG, Soulillou JP: Disposition of basiliximab, an interleukin-2 receptor monoclonal antibody, in recipients of mismatched cadaver renal allografts. Transplantation 64: 1701–1705, 1997
Mould DR, Davis CB, Minthorn EA, Kwok DC, Elliott MJ, Luggen ME, Totoritis MC: A population pharmacokinetic-pharmacodynamic analysis of single doses of clenoliximab in patients with rheumatoid arthritis. Clin Pharmacol Ther 66: 246–257, 1999
Chow FS, Benincosa LJ, Sheth SB, Wilson D, Davis CB, Minthorn EA, Jusko WJ: Pharmacokinetic and pharmacodynamic modeling of humanized anti-factor IX antibody (SB 249417) in humans. Clin Pharmacol Ther 71: 235–245, 2002
Dowell JA, Korth-Bradley J, Liu H, King SP, Berger MS: Pharmacokinetics of gemtuzumab ozogamicin, an antibody-targeted chemotherapy agent for the treatment of patients with acute myeloid leukemia in first relapse. J Clin Pharmacol 41: 1206–1214, 2001
Everitt DE, Davis CB, Thompson K, DiCicco R, Ilson B, Demuth SG, Herzyk DJ, Jorkasky DK: The pharmacokinetics, antigenicity, and fusion-inhibition activity of RSHZ19, a humanized monoclonal antibody to respiratory syncytial virus, in healthy volunteers. J Infect Dis 174: 463–469, 1996
Loh A, Sgouros G, O'Donoghue JA, Deland D, Puri D, Capitelli P, Humm JL, Larson SM, Old LJ, Divgi CR: Pharmacokinetic model of iodine-131-G250 antibody in renal cell carcinoma patients. J Nucl Med 39: 484–489, 1998
Tokuda Y, Watanabe T, Omuro Y, Ando M, Katsumata N, Okumura A, Ohta M, Fujii H, Sasaki Y, Niwa T, Tajima T: Dose escalation and pharmacokinetic study of a humanized anti-HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. Br J Cancer 81: 1419–1425, 1999
Washington CB, Lieberman G, Liu P, Fox JA, Bruno R: A population pharmacokinetic (PK) model for trastuzumab (T) following weekly dosing. Clin Pharmacol Ther 70: P12, 2002
Schmidt A, Muller D, Mersmann M, Wuest T, Gerlach E, Garin-Chesa P, Rettig WJ, Pfizenmaier K, Moosmayer D: Generation of human high-affinity antibodies specific for the fibroblast activation protein by guided selection. Eur J Biochem 268: 1730–1738, 2001
Waldmann TA, Strober W: Metabolism of immunoglobulins. Prog Allergy 13: 1–110, 1969
Morell A, Terry WD, Waldmann TA: Metabolic properties of IgG subclasses in man. J Clin Invest 49: 673–680, 1970
Ghetie V, Popov S, Borvak J, Radu C, Matesoi D, Medesan C, Ober RJ, Ward ES: Increasing the serum persistence of an IgG fragment by random mutagenesis. Nat Biotechnol 15: 637–640, 1997
Kovarik JM, Nashan B, Neuhaus P, Clavien PA, Gerbeau C, Hall ML, Kom A: A population pharmacokinetic screen to identify demographic-clinical covariates of basiliximab in liver transplantation. Clin Pharmacol Ther 69: 201–209, 2001
Wiseman LR, Faulds D: Daclizumab: A review of its use in the prevention of acute rejection in renal transplant recipients. Drugs 58: 1029–1042, 1999
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kloft, C., Graefe, EU., Tanswell, P. et al. Population Pharmacokinetics of Sibrotuzumab, A Novel Therapeutic Monoclonal Antibody, in Cancer Patients. Invest New Drugs 22, 39–52 (2004). https://doi.org/10.1023/B:DRUG.0000006173.72210.1c
Issue Date:
DOI: https://doi.org/10.1023/B:DRUG.0000006173.72210.1c