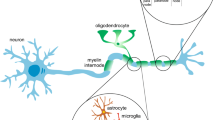
Abstract
Astrocytes are now known to be involved in the most integrated functions of the central nervous system. These functions are not only necessary for the normally working brain but are also critically involved in many pathological conditions, including stroke. Astrocytes may contribute to damage by propagating spreading depression or by sending proapoptotic signals to otherwise healthy tissue via gap junction channels. Astrocytes may also inhibit regeneration by participating in formation of the glial scar. On the other hand, astrocytes are important in neuronal antioxidant defense and secrete growth factors, which probably provide neuroprotection in the acute phase, as well as promoting neurogenesis and regeneration in the chronic phase after injury. A detailed understanding of the astrocytic response, as well as the timing and location of the changes, is necessary to develop effective treatment strategies for stroke patients.
Similar content being viewed by others
REFERENCES
Ameriso, S. F. and Sahai, S. 1997. Mechanisms of ischemia in in situ vascular occlusive disease.
Pulsinelli, W. A., Jacewicz, M., Levy, D. E., Petito, C. K., and Plum, F. 1997. Ischemic brain injury and the therapeutic window. Ann. N. Y. Acad. Sci. 835:187–193.
Lipton, P. 1999. Ischemic cell death in brain neurons. Physiol. Rev. 79:1431–1568.
Legos, J. J. 2002. Pharmacological interventions for stroke: failures and future. Expert. Opin. Investig. Drugs 11:603–614.
Wardlaw, J. M., Warlow, C. P., and Counsell, C. 1997. Systematic review of evidence on thrombolytic therapy for acute ischaemic stroke. Lancet 350:607–614.
Hacke, W., Kaste, M., Fieschi, C., von Kummer, R., Davalos, A., Meier, D., Larrue, V., Bluhmki, E., Davis, S., Donnan, G., Schneider, D., Diez-Tejedor, E., and Trouillas, P. 1998. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australian Acute Stroke Study Investigators Lancet 352:1245–1251.
Kaste, M., Thomanssen, L., Grond, M., Hacke, W., Holtåas, S., Lindley, R. I., Rone, R. O., Wahlgren, N. G., and Wardlaw, J. M. 2000. Thrombolysis for acute ischemic stroke: a consensus statement of the 3rd Karolinska stroke update. Stroke 32:2717–2718.
Ringleb, P. A., Schellinger, P. D., Schranz, C., and Hacke, W. 2002. Thrombolytic therapy with 3 to 6 hours after onset of ischemic stroke. Useful or harmful? Stroke 33:1437–1441.
Cochrane Database Syst. Rev 2002;(1):CD000197. Organised inpatient (stroke unit) care for stroke. Stroke Unit Trialists' Collaboration.
Hertz, L., Yu, A. C., Kala, G., and Schousboe, A. 2000. Neuronal-astrocytic and cytosolic-mitochondrial metabolite trafficking during brain activation, hyperammonemia and energy deprivation. Neurochem. Int. 37:83–102.
Araque, A., Carmignoto, G., and Haydon, P. G. 2001. Dynamic signalling between astrocytes and neurons. Annu. Rev. Physiol. 63:795–813.
Haydon, P. G. 2001. GLIA:listening and talking to the synapse. Nat. Rev. Neurosci. 2:185–193.
Kirchhoff, F., Dringen, R., and Giaume, C. 2001. Pathways of neuron-astrocyte interactions and their possible role in neuro-protection. Eur. Arch. Psychiatry Clin. Neurosci. 251:159–169.
Forsyth, R. J. 1996. Astrocytes and the delivery of glucose from plasma to neurons. Neurochem. Int. 28:231–241.
Nilsson M., Hansson, E., and Rönnbäck, L. 1991. Adrenergic and 5–HT2 receptors on the same astroglial cell: A microspectrofluorimetric study on cytosolic Ca2+ responses in single cells in primary culture. Brain Res. Dev. Brain Res. 63:33–41.
Nilsson M., Eriksson, P. S., Rönbbäck, L., and Hansson, E. 1993. GABA induces Ca2+ transients in astrocytes. Neuroscience 54:605–614.
Verkhratsky, A. and Kettenmann, H. 1996. Calcium signalling in glial cells. Trends Neurosci. 19:346–352.
Porter, J. T. and McCarthy, K. D. (1997) Astrocytic neurotransmitter receptors in situ and in vivo. Prog. Neurobiol. 51: 439–455.
Ventura, R. and Harris, K. M. 1999. Three-dimensional relationships between hippocampal synapses and astrocytes. J. Neurosci. 19:6897–6906.
Bezzi, P., Carmignoto, G., Pasti, L., Vesce, S., Rossi, D., Rizzini, B. L., Pozzan, T. and Volterra, A. 1998. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 391:281–285.
Duffy, S. and MacVicar, B. A. 1995. Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. J. Neurosci. 15:5535–5550.
Shelton, M. K. and McCarthy, K. D. 2000. Hippocampal astrocytes exhibit Ca2+-elevating muscarinic cholinergic and histaminergic receptors in situ. J. Neurochem. 74:555–563.
Kang, J., Jiang, L., Goldman, S. A., and Nedergaard, M. 1998. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat. Neurosci. 1:683–692.
Parpura, V., Basarsky, T. A., Liu, F., Jeftinija, K., Jeftinija, S., and Haydon, P. G. 1994. Glutamate-mediated astrocyte-neuron signalling. Nature 369:744–747.
Muyderman, H., Angehagen, M., Sandberg, M., Bjorklund, U., Olsson, T., Hansson, E., and Nilsson, M. 2001. Alpha 1–adrenergic modulation of metabotropic glutamate receptor-induced calcium oscillations and glutamate release in astrocytes. J. Biol. Chem. 276:46504–46514.
Araque, A., Parpura, V., Sanzgiri, R. P., and Haydon, P. G. 1998. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur. J. Neurosci. 10:2129–2142.
Rouach, N., Avignone, E., Meme, W., Koulakoff, A., Venance, L., Blomstrand, F., and Giaume, C. 2002. Gap junctions and connexin expression in the central nervous system. Biol. Cell. In press.
Tabernero, A., Giaume, C., and Medina, J. M. 1996. Endothelin-1 regulates glucose utilization in cultured astrocytes by controlling intercellular communication through gap junctions. Glia 16:187–195.
Charles, A. and Giaume, C. 2002. Intercellular calcium waves in astrocytes: underlying mechanisms and functional significance. Page 110–126, in Volterra, A., Haydon, P., and Magistretti, P. (eds), In Tripartite Synapses: Synaptic Transmission with Glia, Oxford University Press, London.
Enkvist, M. O. and McCarthy, K. D. 1994. Astroglial gap junction communication is increased by treatment with either glutamate or high K+ concentration. J. Neurochem. 62:489–495.
Blomstrand, F., Khatibi, S., Muyderman, H., Hansson, E., Olsson, T., and Ronnback, L. 1999. 5–Hydroxytryptamine and glutamate modulate velocity and extent of intercellular calcium signalling in hippocampal astroglial cells in primary cultures. Neuroscience 88:1241–1253.
Lipton, P. 1999. Ischemic cell death in brain neurons. 79: 1431–1568.
Folbergrová, J., Memezawa, H., Smith, M.-L., and Siesjö, B. K. 1992. Focal and perifocal changes in tissue energy state during middle cerebral artery occlusion in normo-and hyperglycemic rats. J. Cereb. Blood Flow Metab. 12:25–33.
Kuroda, S., Katsura, K.-I., Tsuchidate, R., and Siesjö, B. K. 1996. Secondary bioenergetic failure after transient focal ischemia is due to mitochondrial injury. Acta Physiol. Scand. 156:149–150.
Anderson, M. F. and Sims, N. R. 1999. Mitochondrial respiratory function and cell death in focal cerebral ischaemia. J. Neurochem. 73:1189–1199.
Sims, N. R. and Zaidan, E. 1995. Biochemical changes associated with selective neuronal death following short-term cerebral ischemia. Int. J. Biochem. Cell Biol. 27:531–550.
Hossmann, K.-A. 1994. Viability thresholds and the penumbra of focal ischemia. Ann. Neurol. 36:557–565.
Kaplan, B., Brint, S., Tanabe, J., Jacewicz, M., Wang, X.-J., and Pulsinelli, W. A. 1991. Temporal thresholds for neocortical infarction in rats subjected to reversible focal cerebral ischemia. Stroke 22:1032–1039.
Buchan, A. M., Xue, D., and Slivka, A. 1992. A new model of temporary focal neocortical ischemia in the rat. Stroke 23: 273–279.
Memezawa, H., Smith, M.-L., and Siesjö, B. K. 1992. Penumbral tissues salvaged by reperfusion following middle cerebral artery occlusion in rats. Stroke 23:552–559.
Garcia, J. H., Liu, K.-F., and Ho, K.-L. 1995. Neuronal necrosis after middle cerebral artery occlusion in Wistar rats progresses at different time intervals in the caudoputamen and the cortex. Stroke 26:636–642.
Li, Y., Chopp, M., Jiang, N., Zhang, Z. G., and Zaloga, C. 1995. Induction of DNA fragmentation after 10 to 120 minutes of focal cerebral ischemia in rats. Stroke 26:1252–1257.
Markgraf, C. G., Velayo, N. L., Johnson, M. P., McCarty, D. R., Medhi, S., Koehl, J. R., Chmielewski, P. A., and Linnik, M. D. 1998. Six-hour window of opportunity for calpain inhibition in focal cerebral ischemia in rats. Stroke 29:152–158.
Yoshimoto, T. and Siesjö, B. K. 1999. Posttreatment with the immunosuppressant cyclosporin A in transient focal ischemia. Brain Res. 839:283–291.
Marrif, H. and Juurlink, B. H. 1999. Astrocytes respond to hypoxia by increasing glycolytic capacity. J. Neurosci. Res. 57: 255–260.
Garcia, J. H., Yoshida, Y., Chen, H., Li, Y., Zhang, Z. G., Lian, J., Chen, S., and Chopp, M. 1993. Progression from ischemic injury to infarct following middle cerebral artery occlusion in the rat. Am. J. Pathol. 142:623–635.
Liu, D., Smith, C. L., Barone, F. C., Ellison, J. A., Lysko, P. G., Li, K., and Simpson, I. A. 1999. Astrocytic demise precedes delayed neuronal death in focal ischemic rat brain. Mol. Brain Res. 68:29–41.
Hagberg, A., Qu, H., Saether, O., Unsgard, G., Haraldseth, O., and Sonnewald, U. 2001. Differences in neurotransmitter synthesis and intermediary metabolism between glutamatergic and GABAergic neurons during 4 hours of middle cerebral artery occlusion in the rat: The role of astrocytes in neuronal survival. J. Cereb. Blood Flow Metab. 21:1451–1463.
Kimelberg, H. K., Goderie, S. K., Higman, S., Pang, S., and Waniewski, R. A. 1990. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J. Neurosci. 10: 1583–1591.
Landis, D. M. 1994. The early reactions of non-neuronal cells to brain injury. Annu. Rev. Neurosci. 17:133–151.
Aschner, M. 1998. Astrocytic functions and physiological reactions to injury: the potential to induce and/or exacerbate neuronal dysfunction—a forum position paper. Neurotoxicology 19:7–17.
Kimelberg, H. C. 2000. Cell volume in the CNS: Regulation and implication for central nervous system pathology. Neuroscientist 6:13–24.
Syková, E. 2001. Glial diffusion barriers during aging and pathological states. Prog. Brain Res. 132:339–363.
Ayata, C. and Ropper, A. H. 2002. Ischemic brain oedema. J. Clin. Neurosci. 9:113–124.
Syková, E. 1997. The extracellular space in the CNS: its regulation, volume and geometry in normal and pathological neuronal function. Neuroscientist 3:28–41.
Takahashi, K., Greenberg, J. H., Jackson, P., Maclin, K., and Zhang, J. 1997. Neuroprotective effects of inhibiting poly(ADP-ribose) synthetase on focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 17:1137–1142.
Hudspith, M. J. 1997. Glutamate: a role in normal brain function, anaesthesia, analgesia and CNS injury. Br. J. Anaesth. 78:731–747.
Guthrie, P. B., Knappenberger, J., Segal, M., Bennett, M. V., Charles, A. C., and Kater, S. B. 1999. ATP released from astrocytes mediates glial calcium waves. J. Neurosci. 19:520–528.
Innocenti, B., Parpura, V., and Haydon, P. G. 2000. Imaging extracellular waves of glutamate during calcium signaling in cultured astrocytes. J. Neurosci. 20:1800–1808.
Martínez, A. D. and Sáez, J. C. 2000. Regulation of astrocytes gap junctions by hypoxia-reoxygenation. Brain Res. Rev. 32:250–258.
Cotrina, M. L., Kang, J., Lin, J. H., Bueno, E., Hansen, T. W., He, L., Liu, Y., and Nedergaard M. 1998. Astrocytic gap junctions remain open during ischemic conditions. J. Neurosci. 18:2520–2537.
Lin, J. H., Weigel, H., Cotrina, M. L., Liu, S., Bueno, E., Hansen, A. J., Hansen, T. W., Goldman, S., and Nedergaard, M. 1998. Gap-junction-mediated propagation and amplification of cell injury. Nat. Neurosci. 1:494–500.
Li, Y., Chopp, M., Jiang, N., Yao, F., and Zaloga, C. 1995. Temporal profile of in situ DNA fragmentation after transient middle cerebral artery occlusion in the rat. J. Cereb. Blood Flow Metab. 15:389–397.
Li, Y., Chopp, M., Jiang, N., and Zaloga, C. 1995. In situ detection of DNA fragmentation after focal cerebral ischemia in mice. Mol. Brain Res. 28:164–168.
Budd, S. L. and Lipton, S. A. 1998. Calcium tsunamis: do astrocytes transmit cell death messages via gap junctions during ischemia? Nat. Neurosci. 1:431–432.
Rami, A., Volkmann, T., and Winckler, J. 2001. Effective reduction of neuronal death by inhibiting gap junctional intercellular communication in a rodent model of global transient cerebral ischemia. Exp. Neurol. 170:297–304.
Rawanduzy, A., Hansen, A., Hansen, T. W., and Nedergaard, M. 1997. Effective reduction of infarct volume by gap junction blockade in a rodent model of stroke. J. Neurosurg. 87:916–920.
Saito, R., Graf, R., Hubel, K., Fujita, T., Rosner, G., and Heiss, W. D. 1997. Reduction of infarct volume by halothane: effect on cerebral blood flow or perifocal spreading depression-like depolarisations. J. Cereb. Blood Flow Metab. 17:857–864.
Siushansian, R., Bechberger, J. F., Cechetto, D. F., Hachinski, V. C., and Naus, C. C. 2001. Connexin43 null mutation increases infarct size after stroke. J. Comp. Neurol. 440:387–394.
Blanc, E. M., Bruce-Keller, A. J., and Mattson, M. P. 1998. Astrocytic gap junction communication decreases neuronal vulnerability to oxidative stress-induced disruption of Ca2+ homeostasis and cell death. J. Neurochem. 70:958–970.
Nedergaard, M. and Astrup, J. 1986. Infarct rim: effect of hyperglycemia on direct current potential and [14C]2–deoxyglucose phosphorylation. J. Cereb. Blood Flow Metab. 6:607–615.
Gill, R., Nordholm, L., and Lodge, D. 1992. The neuroprotective actions of 2,3–dihydroxy-6–nitro-7–sulfamoyl-benzo(F)quinozaline (NBQX) in a rat focal ischaemic model. Brain Res. 580: 35–43.
Nedergaard, M. and Hansen, A. J. 1998. Spreading depression is not associated with neuronal injury in the normal brain. Brain Res. 449:395–398.
Iijima, T., Mies, G., and Hossmann, K. A. 1992. Repeated negative DC deflections in rat cortex following middle cerebral artery occlusion are abolished by MK-801: effect on volume of ischemic injury. J. Cereb. Blood Flow Metab. 12:727–733.
Chen, Q., Chopp, M., Bodzin, G., and Chen, H. 1993. Temperature modulation of cerebral depolarization during focal cerebral ischemia in rats: Correlation with ischemic injury. J. Cereb. Blood Flow Metab. 13:389–394.
Mies, G. 1993. Inhibition of protein synthesis during repetitive cortical spreading depression. J. Neurochem. 60:360–363.
Busch, E., Gyngell, M. L., Eis, M., Hoehn-Berlage, M., and Hossmann, K. A. 1996. Potassium-induced cortical spreading depressions during focal cerebral ischemia in rats: contribution to lesion growth assessed by diffusion-weighted NMR and biochemical imaging. J. Cereb. Blood Flow Metab. 16:1090–1099.
Takano, K., Latour, L. L., Formato, J. E., Carano, R. A., Helmer, K. G., Hasegawa, Y., Sotak, C. H., and Fisher, M. 1996. The role of spreading depression in focal ischemia evaluated by diffusion mapping. Ann. Neurol. 39:308–318.
Martins-Ferreira, H., Nedergaard, M., and Nicholson, C. 2000. Perspectives on spreading depression. Brain Res. Rev. 32:215–234.
Basarsky, T. A., Duffy, S. N., Andrew, R. D., and MacVicar, B. A. 1998. Imaging spreading depression and associated intracellular calcium waves in brain slices. J. Neurosci. 18: 7189–7199.
Kunkler, P. E. and Kraig, R. P. 1998. Calcium waves preceed electrophysiological changes of spreading depression in hippocampal cultures. J. Neurosci. 18:3416–3425.
Sugaya, E., Takato, M., and Noda, Y. 1975. Neuronal and glial activity during spreading depression in cerebral cortex of cat. J. Neurophysiol. 38:822–841.
Largo, C., Cuevas, P., and Herreras, O. 1996. Is glia disfunction the initial cause of neuronal death in ischemic penumbra? Neurol. Res. 18:445–448.
Floyd, R. A. 1999. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc. Soc. Exp. Biol. Med. 222:236–245.
Bains, J. S. and Shaw, C. A. 1997. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res. Rev. 25:335–358.
Schulz, J. B., Lindenau, J., Seyfried, J., and Dichgans, J. 2000. Glutathione, oxidative stress and neurodegeneration Eur. J. Biochem. 267:4904–4911.
Chan, P. H. 1996. Role of oxidants in ischemic brain damage. Stroke 27:1124–1129.
Piantadosi, C. A. and Zhang, J. 1996. Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke 27:327–331.
Kuroda, S. and Siesjö, B. K. 1997. Reperfusion damage following focal ischemia: pathophysiology and therapeutic windows. Clin. Neurosci. 4:199–212.
Nowicki, J. P., Duval, D., Poignet, H., and Scatton, B. 1991. Nitric oxide mediates neuronal death after focal cerebral ischemia in the mouse. Eur. J. Pharmacol. 204:339–340.
Ranjan, A., Theodore, D., Haran, R. P., and Chandy, M. J. 1993. Ascorbic acid and focal cerebral ischaemia in a primate model. Acta Neurochir. (Wien.) 123:87–91.
Dawson, D. A., Graham, D. I., McCulloch, J., and Macrae, I. M. 1994. Anti-ischaemic efficacy of a nitric oxide synthase inhibitor and a N-methyl-D-aspartate receptor antagonist in models of transient and permanent focal cerebral ischaemia. Br. J. Pharmacol. 113:247–253.
Baker, K., Marcus, C. B., Huffman, K., Kruk, H., Malfroy, B., and Doctrow, S. R. 1998. Synthetic combined superoxide dismutase catalase mimetics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury. J. Pharm. Exp. Ther. 284:215–221.
Fukuyama, N., Takizawa, S., Ishida, H., Hoshiai, K, Shinohara, Y., and Nakazawa, H. 1998. Peroxynitrite formation in focal cerebral ischemia-reperfusion in rats occurs predominantly in the peri-infarct region. J. Cerebr. Blood Flow Metab. 18:123–129.
Heales, S. J. R., Bolaños, J. P., Stewart V. C., Brookes, P. S., Land, J. M., and Clark, J. B. 1999. Nitric oxide, mitochondria and neurological disease. Biochim. Biophys. Acta 1410:215–228.
Dringen, R. 2000. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 62:649–671.
Dringen, R., Pfeiffer, B., and Hamprecht, B. 2002. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J. Neurosci. 19:562–569.
Iwata-Ichikawa, E., Kondo, Y., Miyazaki, I., Asanuma, M., and Ogawa, N. 1999. Glial cells protect neurons against oxidative stress via transcriptional up-regulation of the glutathione synthesis. J. Neurochem. 72:2334–2344.
Chen, Y., Vartiainen, N. E., Ying, W., Chan, P. H., Koistinaho, J., and Swanson, R. A. 2001. Astrocytes protect neurons from nitric oxide toxicity by a glutathione-dependent mechanism. J. Neurochem. 77:1601–1610.
Wallin, C., Puka-Sundvall, M., Hagberg, H., Weber, S. G., and Sandberg, M. 2000. Alterations in glutathione and amino acid concentrations after hypoxia-ischemia in the immature rat brain. Dev. Brain Res. 125:51–60.
Uemura, Y., Miller, J. M., Matson, W. R., and Beal, M. F. 1991. Neurochemical analysis of focal ischemia in rats. Stroke 22: 1548–1553.
Gotoh, O., Yamamoto, M., Tamura, A., and Sano, K. 1994. Effect of YM737, a new glutathione analogue, on ischemic brain edema. Acta Neurochir. Suppl. 60, 318–320.
Anderson, M. F. and Sims, N. R. 2002. The effects of focal ischemia and reperfusion on the glutathione content of mitochondria from rat brain subregions. J. Neurochem. 81:541–549.
Mizui, T., Kinouchi, H., and Chan, P. H. 1992. Depletion of brain glutathione by buthionine sulfoximine enhances cerebral ischemic injury in rats. Am. J. Physiol. 262:H313–H317.
Fernández-Checa, J. C., García-Ruiz, C., Ookhtens, M., and Kaplowitz, N. 1991. Impaired uptake of glutathione by hepatic mitochondria from chronic ethanol-fed rats. Tracer kinetic studies in vitro and in vivo and susceptibility to oxidant stress. J. Clin. Invest. 87:397–405.
García-Ruiz, C., Morales, A., Ballesta, A., Rodes, J., Kaplowitz, N., and Fernández-Checa, J. C. 1994. Effect of chronic ethanol feeding on glutathione and functional integrity of mitochondria in periportal and perivenous rat hepatocytes. J. Clin. Invest. 94:193–201.
Collel, A., García-Ruiz, C., Miranda, M., Ardite, E., Mári, M., Morales, A., Corrales, F., Kaplowitz, N., and Fernández-Checa, J. C. 1998. Selective glutathione depletion of mitochondria by ethanol sensitises hepatocytes to tumour necrosis factor. Gastroenterology 115:1541–1551.
Wüllner, U., Seyfried, J., Groscurth, P., Beinroth, S., Gleichmann, M., Heneka, M., Löschmann, P. A., Schulz, J. B., Weller, M., and Klockgether, T. 1999. Glutathione depletion and neuronal cell death: the role of reactive oxygen intermediates and mitochondrial function. Brain Res. 826:53–62.
Schallert, T., Leasure, J. L., and Kolb, B. 2000. Experience-associated structural events, subependymal cellular proliferative activity, and functional recovery after injury to the central nervous system. J. Cereb. Blood Flow Metab. 20:1513–1528.
Dahlqvist, P., Zhao, L., Johansson, I. M., Mattsson, B., Johansson, B. B., Seckl, J. R., and Olsson, T. 1999. Environmental enrichment alters nerve growth factor-induced gene A and glucocorticoid receptor messenger RNA expression after middle cerebral artery occlusion in rats. Neuroscience 93:527–535.
Liepert, J., Bauder, H., Miltner, W. H. R., Taub, E., and Weiller, C. 2000. Treatment-induced cortical reorganization after stroke in humans. Stroke 31:1210–1216.
Johansson, B. B. and Belichenko, P. V. 2002. Neuronal plasticity and dentritic spines: effect of environmental enrichment on intact and postischemic rat brain. J. Cereb. Blood Flow Metab. 22:89–96.
Komitova, M., Perfilieva, E., Mattsson, B., Eriksson, P. S., and Johansson, B. B. 2002. Effects of cortical ischemia and postischemic environmental enrichment on hippocampal cell genesis and differentiation in the adult rat. J. Cereb. Blood Flow Metab. 22:852–860.
Ridet, J. L., Malhotra, S. K., Privat, A., and Gage, F. H. 1997. Reactive astrocytes: cellular and molecular cues to biological functions. Trends Neurosci. 20:570–577.
Little, A. R. and O'Callaghan, J. P. 2001. Astrogliosis in the adult and developing CNS: is there are role for proinflammatory cytokines. NeuroToxicology 22:607–618.
Clarke, S. R., Shetty, A. K., Bradley, J. L., and Turner, D. A. 1994. Reactive astrocytes express the embryonic intermediate neurofilament nestin. Neuroreport 5:1885–1888.
Eng, L. F. and Ghirnikar, R. S. 1994. GFAP and astrogliosis. Brain Pathol. 4:229–237.
Holmin, S., Almqvist, P., Lendahl, U., and Mathiesen, T. 1997. Adult nestin-expressing subependymal cells differentiate to astrocytes in response to brain injury. Eur. J. Neurosci. 9:65–75.
Pixley, S. K. and De Vellis, J. 1984. Transition between radial glia and mature astrocytes studied with a monoclonal antibody to vimentin. Brain Res. 15:201–209.
Lendahl, V., Zimmerman, L. B., and McKay, R. D. G. 1990. CNS stem cells express a new class of intermediate filament protein. Cell 60:585–595.
Sancho-Tello, M., Vallés, S., Montoliu, C., Renau-Piqueras, J., and Guerri, C. 1995. Developmental pattern of GFAP and vimentin expression in rat brain and radial glial cultures. Glia 15:157–166.
Kajihara, H., Tsutsumi, E., Kinoshita, A., Nakano, J., Takagi, K., and Takeo, S. 2001. Activated astrocytes with glycogen accumulation in ischemic penumbra during the early stage of brain infarction: immunohistochemical and electron microscopic studies. Brain Res. 909:92–101.
Cramer, S. C. and Chopp, M. 2000. Recovery recapitulates ontogeny. Trends Neurosci. 23:265–271.
Luskin, M. B. 1993. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain sub-ventricular zone. Neuron 11:173–189.
Lois, C. and Alvarez-Buylla, A. 1994. Long-distance neuronal migration in the adult mammalian brain. Science 264:1145–1148.
Eriksson, P. S., Perfilieva, E., Björk-Eriksson, T., Alborn, A.-M., Nordborg C., and Gage, F. H. 1998. Neurogenesis in the adult human hippocampus. Nature Med. 11:1313–1317.
Arvidsson, A., Kokaia, Z., and Lindvall, O. 2001. N-Methyl-D-aspartate receptor-mediated increase of neurogenesis in adult rat dentate gyrus following stroke. Eur. J. Neurosci. 14:10–18.
Jiang, W., Gu, W., Brännström, T., Rosqvist R., and Wester, P. 2001. Cortical neurogenesis in adult rats after transient middle cerebral artery occlusion. Stroke 32:1201–1207.
Jin, K, Minami, M., Lan, J. Q., Mao, X. O., Batteur, S., Simon, R. P., and Greenberg, D. A. 2001. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc. Natl. Acad. Sci. USA 98: 4710–4715.
Kee, N. J., Preston, E., and Wojtowicz, J. M. 2001. Enhanced neurogenesis after transient global ischemia in the dentate gyrus of the rat. Exp. Brain Res. 136:313–320.
Zhang, R. L., Zhang, Z. G., Zhang, L., and Chopp, M. 2001. Proliferation and differentiation of progenitor cells in the cortex and subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience 105:33–41.
Palmer, T. D., Markakis, E. A., Willhoite, A. R., Safar, F., and Gage, F. H. 1999. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cell from diverse regions of adult CNS. J. Neurosci. 19:8487–8497.
Palmer, T. D., Ray, J., and Gage, F. H. 1995. Fibroblast growth factor-2 responsive neuronal progenitors reside in proliferating and quiescent regions of the adult rodent brain. Mol. Cell. Neurosci. 6:474–486.
Gu, W., Brännström, T., and Wester, P. 2000. Cortical neurogenesis in adult rats after reversible photothrombotic stroke. J. Cereb. Blood Flow Metab. 20:1166–1173.
Kuhn, H. G., Winkler, J., Kempermann, G., Thal, L. J., and Gage, F. H. 1997. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J. Neurosci. 17:5820–5829.
Åberg, A. I., Åberg, D., Hedbäcker, H., Oscarsson, J., and Eriksson, P. S. 2000. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J. Neurosci. 20:2896–2903.
Lim, D. A. and Alvarez-Buylla, A. 1999. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc. Natl. Acad. Sci. USA 96:7526–7531.
Song, H.-J., Stevens, C. F., and Gage, F. H. 2002. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nature Neurosci. 5:438–445.
Alonso, G. 2001. Proliferation of progenitor cells in the adult rat brain correlates with the presence of vimentin-expressing astrocytes. Glia 34:253–266.
Song, H., Stevens, C. F., and Gage, F. H. 2002. Astroglia induce neurogenesis from adult neural stem cells. Nature 417:39–44.
Doetsch, F., García-Verdugo, J. M., and Alvarez-Buylla, A. 1997. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 17:5046–5061.
Doetsch, F., Caille, I., Lim, D. A., Garcia-Verdugo, J. M., and Alvarez-Buylla, A. 1999. Subventricular zone astrocytes are neuronal stem cells in the adult mammalian brian. Cell 97:703–716.
Seri, B., García-Verdugo, J. M., McEwen, B. S., and Alvarez-Buylla, A. 2001. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J. Neurosci. 21:7153–7160.
Alvarez-Buylla, A., Seri, B., and Doetsch, F. 2002. Identification of neural stem cells in the adult vertebrate brain. Brain Res. Rev. 57:751–758.
Åberg, M. A. I., Hellgren, G., Lindell, K., Rosengren, L., MacLennan, A. J., Carlsson, B., and Eriksson, P. S. 2001. CNTF induces gliogenesis via the JAK-STAT-Tis11 signaling pathway in adult CNS progenitor cells. Mol. Cell Neurosci. 17: 426–443.
Lin, T.-N., Wang, P. Y., Chi, S. I., and Kuo, J. S. 1998. Differential regulation of ciliary neurotrophic factor (CNTF) and CNTF receptor alpha (CNTFR alpha) expression following focal cerebral ischemia. Mol. Brain Res. 55:71–80.
Fawcett, J. W. and Asher R. A. 1999. The glial scar and central nervous system repair. Brain Res. Bull. 49:377–391.
McGraw, J., Hiebert, G. W., and Steeves, J. D. 2001. Modulating astrogliosis after neurotrauma. J. Neurosci. Res. 63: 109–115.
Pekny, M., Johansson, C. B., Eliasson, C., Stakeberg, J., Wallen, A., Perlmann, T., Lendahl, U., Betsholtz, C., Berthold, C. H., and Frisen, J. 1999. Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J. Cell Biol. 145:503–514.
Menet, V., Gimenez, Y. R., Sandillon, F., and Privat, A. 2000. GFAP null astrocytes are a favourable substrate for neuronal survival and neurite growth. Glia 31:267–272.
Costa, S., Planchenault, T., Charriere-Bertrand, C., Mouchel, Y., Fages, C., Juliano, S., Lefrancois, T., Barlovatz-Meimon, G., and Tardy, M. 2002. Astroglial permissivity for neurite outgrowth in neuron-astrocyte cocultures depends on regulation of laminin bioavailability. Glia 37:105–113.
Hamberger, A. and Hyden, H. 1963. Inverse enzymatic changes in neurons and glia during increased function and hypoxia. J. Cell Biol. 6:521–525.
Blomstrand, C., Hallen, O., Hamberger, A., and Jarlstedt, J. 1966. Quantitative cytochemical aspects on the mechanism of central compensation after unilateral vestibular neurotomy. Acta Otolaryngol. 61:113–120.
Blomstrand, C., Hallen, O., Hamberger, A., and Jarlstedt, J. 1966. Effects of unilateral warm and cold water irrigation in the outer ear of rabbits on isolated nerve cells from the lateral vestibular nucleus and cerebellum. Acta Otolaryngol. 61:527–535.
Blomstrand, C., Hallen, O., Hamberger, A., and Jarlstedt, J. 1970. Effect of cerebellectomy upon the cytochemistry of neurons in the lateral vestibular nucleus 3. Cytochemical response to unilateral vestibular stimulation after midline section of the brain stem. Brain Res. 19:427–432.
Blomstrand, C. and Hamberger, A. 1969. Protein turnover in cell-enriched fractions from rabbit brain. J. Neurochem. 16:1401–1407.
Blomstrand, C. and Hamberger, A. 1970. Amino acid incorporation in vitro into protein of neuronal and glial cell-enriched fractions. J. Neurochem. 17:1187–1195.
Henn, F. A. and Hamberger, A. 1971. Glial cell function: uptake of transmitter substances. Proc Natl. Acad. Sci. USA 68:2686–2690.
Blomstrand, C. 1970. Effect of hypoxia on protein metabolism in neuron-and neuroglia cell-enriched fractions from rabbit brain. Exp. Neurol. 29:175–188.
Cochrane Database Syst. Rev 2002;(1):CD000197. Organised inpatient (stroke unit) care for stroke. Stroke Unit Trialists' Collaboration.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anderson, M.F., Blomstrand, F., Blomstrand, C. et al. Astrocytes and Stroke: Networking for Survival?. Neurochem Res 28, 293–305 (2003). https://doi.org/10.1023/A:1022385402197
Issue Date:
DOI: https://doi.org/10.1023/A:1022385402197