Abstract
In the field of nuclear medicine, multiagent imaging can disclose enhanced expression of specific target molecules of neoplastic cells. This molecular information can be combined with the information provided by anatomical imaging. Over the past 20 years the use of radiolabeled somatostatin analogs as high-affinity tracers binding specifically to somatostatin receptors has allowed successful molecular imaging of neuroendocrine neoplasms (NENs), initially with single-photon-emitting radiopharmaceuticals, and subsequently also with positron-emitting radiopharmaceuticals. In this context, whole-body somatostatin receptor scintigraphy has changed the diagnostic and therapeutic approach to patients with NENs; moreover, somatostatin analog positron emission tomography (PET) tracers that allow higher spatial resolution imaging have recently been introduced. Nevertheless, several NENs can also be successfully imaged with radioagents that target the catecholamine pathway. Although radioiodinated meta-iodobenzylguanidine (MIBG) is traditionally the first option for radionuclide imaging and treatment of these NENs, the use of PET with 18F-l-dihydroxyphenylalanine is being increasingly reported, this approach showing several advantages over conventional imaging. Moreover, changes in tumor biology can be characterized by modifications in receptor or transporter expression on the cell membrane, and evidence has recently emerged that NENs expressing glucose transporters are more aggressive than tumors with low expression of glucose transporters. Therefore, [18F]FDG PET could provide prognostic information useful for stratifying patients according to their risk and for planning the correct therapy. The identification of new radiopharmaceuticals specific for different targets will constitute the basis not only of new diagnostic imaging approaches, but also of new therapeutic applications in NENs, besides radiolabeled peptide receptor radionuclide and 131I-MIBG therapy.
Similar content being viewed by others
Introduction
Neuroendocrine neoplasms (NENs) constitute a heterogeneous group of rare tumors that, arising from cells of the neuroendocrine system, are distributed almost ubiquitously in the body. The term “neuroendocrine” is related to the ability of these cells to produce and store amines and peptide hormones produced by both the endocrine system and the central nervous system: gastrin, insulin, serotonin, somatostatin, glucagon, pancreatic polypeptide, vasoactive intestinal peptide (VIP), catecholamines, ACTH, GH, prolactin, FSH, LH, TSH, and PTH. Although NENs differ in their biology and clinical presentation, they share the capability to produce certain biological compounds, such as chromogranins and synaptophysin, which are considered markers of neuroendocrine cells. According to recent guidelines, the immunohistochemical determination of synaptophysin and chromogranin A in the tumor cells is mandatory for a diagnosis of NEN [1].
NENs frequently express a high density of somatostatin receptors (SSTRs) on cell membranes. Somatostatin, a small cyclic peptide which circulates in the blood in two biologically active forms (somatostatin-14 and somatostatin-28), plays an important role in modulating neurotransmission and secretion, and in controlling proliferation of both normal and tumor cells. Six SSTR subtypes have been identified by molecular analysis (sstr1, sstr2a, sstr2b, sstr3, sstr4, and sstr5), each of which exerts its action by inhibiting adenylyl cyclase activity. Different NENs, as well as different tumor lesions (such as primary site lesions and distant metastases) in the same patient, can express various proportions of these subtypes. The predominant expression of sstr2 on the neuroendocrine cells of the pancreas (α cells), gastrointestinal tract and adrenals is the basis for the successful clinical application of somatostatin analogs in molecular imaging and in therapy [2]. Instead, sstr1 receptors are more frequently expressed by pancreatic β cells, whereas sstr3 and sstr4 are less commonly expressed, and the sstr5 subtype is more frequently related to specific tumors, such as breast cancer.
According to a “surgical” criterion, NENs can be divided into those arising in neuroendocrine organs [such as pancreatic endocrine tumors, pheochromocytomas (PHEOs), paragangliomas (PGLs) and medullary thyroid cancer], those arising from dispersed neuroendocrine cells [such as pulmonary or gastroenteropancreatic (GEP) NENs], and finally those arising from non-neuroendocrine organs, such as thymic or cutaneous NENs.
Although most NENs are sporadic, they can also be part of multiple endocrine neoplasia type 1 and 2 (MEN1 and MEN2). Hereditary GEP or pulmonary NENs and pituitary adenomas occur in MEN1. Germline mutations in at least five genes have been recognized to be responsible for familial PHEOs: the von Hippel–Lindau gene (VHL) causing von Hippel–Lindau syndrome, the RET gene, which is associated with MEN2, the neurofibromatosis type 1 gene (NF1) which is associated with von Recklinghausen’s disease, and the genes encoding the B and D subunits of mitochondrial succinate dehydrogenase (SDHB and SDHD), which are responsible for familial PGLs and PHEOs.
Since the cell proliferation rate has been shown to provide significant prognostic information, the 2010 World Health Organization (WHO) guidelines have proposed a new classification of GEP NENs based on histopathology and parameters of proliferative activity (Ki67 index and mitotic count). Thus, GEP NENs include two well-differentiated NENs, namely the so-called neuroendocrine tumor G1 (typical carcinoid), the neuroendocrine tumor G2, and a poorly differentiated NEN known as neuroendocrine carcinoma G3 (large-cell or small-cell type). Mixed adenoneuroendocrine carcinoma (a phenotype that is recognizable as both adenocarcinoma and neuroendocrine carcinoma), hyperplastic and preneoplastic lesions are also included in the classification. Although GEP NENs may present with a clinical syndrome related to hormone hypersecretion, symptoms are frequently vague and unrecognized, with the result that the diagnosis is often not made until the metastatic disease stage, when radical treatment with curative intent is no longer possible. In non-functioning NENs, hormone-related manifestations are absent, and clinical symptoms, related to the mass effect, usually occur quite late in the course of the disease.
Gastric NENs classified as type 1 (following atrophic gastritis) or type 2 (in the Zollinger–Ellison syndrome) are associated with hypergastrinemia. Type 3 gastric carcinoids are not associated with hypergastrinemia and are single large lesions, usually with distant metastases. Duodenal NENs frequently secrete gastrin and cause Zollinger–Ellison syndrome, as part of MEN1. Intestinal and appendicular NENs derive from enterochromaffin cells and are frequently non-functioning. On the contrary, metastatic intestinal or appendicular carcinoids are functioning and may present with the carcinoid syndrome, typical or atypical (i.e., cutaneous flushing, diarrhea and abdominal pain), due to the overproduction and release into the systemic circulation of bioactive amines, mostly serotonin and histamine. Colonic carcinoids are frequently large, non-functioning and confer poor prognosis, while rectal carcinoids are small and rarely metastasize. Functioning pancreatic islet-cell tumors may produce insulin, gastrin, VIP, glucagon, somatostatin, etc. Insulinomas are often small, benign lesions causing hypoglycemia. Pancreatic gastrinomas are generally malignant and cause Zollinger–Ellison syndrome (which in 25 % of cases is associated with MEN1). Glucagonomas cause diabetes and a characteristic rash (necrolytic migratory erythema). VIPomas may produce severe watery diarrhea, hypokalemia and achlorhydria (WDHA, or Whipple syndrome). More rarely NENs may secrete ACTH, GHRH, PTH-RP, and even somatostatin [3].
Pulmonary NENs include typical and atypical carcinoids (G1 and G2 neuroendocrine carcinoma), large-cell neuroendocrine carcinoma (LCNEC, or G3 large-cell neuroendocrine carcinoma) and small-cell lung carcinoma (SCLC, or G3 small-cell neuroendocrine carcinoma). Typical carcinoids have a relatively benign, indolent biological course and characteristically present as a centrally located lesion, with signs and symptoms of bronchial obstruction. The carcinoid syndrome occurs after development of liver metastases, which shed active amines into the systemic circulation. Other syndromes related to hormonal overproduction include the Cushing syndrome (ACTH) and acromegaly (GHRH and more rarely GH) [4, 5]. Atypical carcinoids are frequently peripherally located, are functioning, and display a biologically aggressive behavior, spreading through both the lymphatic and the hematogenous routes. LCNEC is an aggressive and rare form, usually extensively metastatic at diagnosis. Finally, SCLC is a frequent and aggressive tumor, with early metastases typically to the brain or bone; although this form is extremely chemosensitive, it also easily relapses and therefore has a poor prognosis. Paraneoplastic syndromes often occur, particularly Cushing syndrome and the syndrome of inappropriate antidiuretic hormone secretion (SIADH) [6–8].
PGLs are rare tumors that arise from parasympathetic and sympathetic paraganglia. The latter can develop in the Zuckerkandl body, the sympathetic plexus of the urinary bladder, the kidneys, the heart and the sympathetic ganglia located in the mediastinum. PGLs arising from adrenal medullary chromaffin cells are also defined as PHEOs. Parasympathetic PGLs are often located in the head and neck region and they do not usually secrete catecholamines [9, 10]. On the contrary, almost all sympathetic PGLs secrete catecholamines and give rise to symptoms resembling those of PHEOs. The most frequent manifestations related to catecholamine hypersecretion are hypertension, tachycardia, headache, pallor and feelings of panic or anxiety. Hyperglycemia, lactic acidosis, weight loss, nausea, fever and flushing may also occur. Arterial blood pressure may be normal in tumors detected incidentally (when the tumor load is limited), or when dopamine rather than catecholamine is preferentially secreted. In rare cases patients may even present lower-than-normal arterial pressure levels (in the presence of prevalent epinephrine secretion) [10].
Principles of nuclear medicine imaging
Several diagnostic techniques can be used for localizing and assessing the extent of NENs, including X-ray computed tomography (CT), CT enterography (CTE), magnetic resonance imaging (MRI), magnetic resonance enterography (MRE), ultrasonography (US), contrast-enhanced US (CEUS), endoscopic US (EUS), intraoperative US (IOUS), selective angiography with hormonal sampling, and nuclear medicine imaging. While morphological imaging techniques (such as US, CT, and MRI) can be useful for localizing the primary tumor (particularly if non-functioning), radionuclide imaging helps in staging the disease (both locoregionally and at distant sites), thus providing important information for therapy decision-making. Nevertheless, no single imaging technique represents the gold standard, and specific sequences of exams are needed for each tumor type. It should also be emphasized that in up to 50 % of NENs the primary site remains unknown.
Nuclear medicine imaging can disclose enhanced expression of specific targets and/or functions of NENs that are characterized by, e.g., abundant cell membrane-bound SSTRs and/or the presence of neuroamine uptake mechanisms. Moreover, since high glucose metabolism of tumors is directly related to cell proliferation (depending on the expression of glucose transporters, GLUT) [11], increased uptake of [18F]FDG by NEN cells is typically related to cell dedifferentiation and high malignancy. On the other hand, as seen with other tumors, [18F]FDG positron emission tomography (PET) does not invariably provide useful information in those NENs that are relatively slow growing (and therefore biologically less aggressive) [12].
Somatostatin receptor imaging
Imaging of NENs involves the use of radiolabeled analogs of somatostatin, In fact, since native somatostatin has a very short biological half-life in plasma, all radiopharmaceuticals utilized for SSTR imaging are based on octreotide, or on similarly long-acting analogs of the human hormone. Moreover, octreotide has the highest affinity for sstr2a-b (K d 0.1–1 nM); its affinity for sstr3 and sstr5 is within the 10–100 nM range, whereas it shows very low affinity for sstr1 and sstr4. This analog is therefore particularly suitable for imaging NENs, since sstr2 is the subtype most frequently expressed in these tumors. SSTR density is markedly elevated in malignant tissues, from 80 to 2,000 fmol/mg protein, whereas the expression of SSTRs within normal neuroendocrine tissues is relatively low. SSTRs are especially overexpressed in certain GEP NENs [13].
123I-[Tyr3]-octreotide was the first radiopharmaceutical used for imaging NENs. However, its relatively short effective half-life and the high background of activity within the abdomen due to release of radioiodide during its metabolic degradation have limited its clinical application. 111In-[DTPA-d-Phe]-octreotide, or 111In-pentetreotide, was subsequently developed; the presence of the radiometal 111In and of the chelating agent DTPA results in greater stability in vivo than is obtained when labeling with radioiodide, while the longer physical half-life of 111In versus 123I allows delayed image acquisition. This radiopharmaceutical (marketed as OctreoScan®, a registered trademark of Mallinckrodt Inc.) binds with high affinity to sstr2, while it shows moderate affinity for sstr3 and sstr5.
Somatostatin receptor imaging in GEP NENs
Somatostatin receptor scintigraphy (SRS) with 111In-pentreteotide is, in general, quite accurate for the detection and staging of GEP NENs, with the exception of the low sensitivity (50–60 %) reported for insulinomas, which are characterized by low expression of sstr2 [14]. The main advantage of SRS is its ability to explore the whole body and to provide important indications for therapy with somatostatin analogs.
At initial diagnosis, it is of paramount importance to integrate the SRS findings with those provided by radiological imaging [15]. Moreover, the use of hybrid SPECT/CT equipment can provide the surgeon with detailed functional and topographic information for tissue sampling and for assessment of resectability [16] (Fig. 1).
By enabling accurate staging of NENs, scintigraphy with 111In-pentreteotide can lead to modification of the therapeutic strategy in up to 53 % of cases. In particular, because of its high sensitivity (61–96 %), SRS is the most accurate imaging modality for the “one-shot” detection of liver and extrahepatic metastases in patients with GEP NENs and may prevent surgery with curative intent in those patients whose tumors have already metastasized [17, 18]. This consideration also applies to patients with insulinomas, as it is known that metastatic insulinoma lesions are more likely than the primary tumor to express abundant SSTRs [19, 20]. However, the small size of liver metastases significantly affects the sensitivity of scintigraphy with 111In-pentreteotide [19–21].
SRS with 111In-pentreteotide is also used to select patients potentially responsive to treatment with unlabeled octapeptide somatostatin analogs, as well as with tumor-targeted radiolabeled somatostatin analogs (the so-called peptide radio-receptor therapy, or PRRT). In fact, intense uptake of 111In-pentreteotide in tumor lesions is associated with a higher probability of response to PRRT [19, 21–24].
It is still debated whether the sensitivity of SSTR imaging is reduced in patients concurrently receiving therapeutic doses of unlabeled octreotide acetate, and the possibility of temporarily suspending this therapy before radiotracer administration should be considered [11, 25].
Like US, CT and MRI, SRS with 111In-pentreteotide is also employed during follow-up to monitor the efficacy of treatment. Changes in functional volumes after treatment, assessed by scintigraphy, have been reported to be more useful than the Response Evaluation Criteria in Solid Tumors (RECIST), and to correlate well with the long-term clinical response [26].
Recently, somatostatin analog PET tracers have been proposed, opening up the possibility of performing SSTR PET, which is characterized by higher spatial resolution, higher signal-to-noise ratios, and easier image quantification than SSTR SPECT [27, 28].
The 68Ga-labeled peptides most commonly used in clinical practice are the DOTA-octapeptides. Among the many different DOTA-octapeptides available worldwide, the most commonly employed are 1,4,7,10-tetraazacyclododecane,1,4,7,10-tetraacetic acid Tyr3 octreotide (DOTA-TOC), 1,4,7,10-tetraazacyclododecane,1,4,7,10-tetraacetic acid Thr8 octreotide (DOTA-TATE) and 1,4,7,10-tetraazacyclododecane,1,4,7,10-tetraacetic acid 1-Nal3 octreotide (DOTA-NOC). Their affinity profiles for SSTRs are well known: -TOC and -TATE show high binding affinity for sstr2, while -NOC recognizes with high-affinity three SSTR subtypes (sstr2, sstr3 and sstr5) [29]. Few studies that have compared different 68Ga-DOTA-octapeptides in the same patients have revealed either comparable diagnostic accuracy [30–32] or better sensitivity for DOTA-NOC versus DOTA-TATE in patients with GEP NENs [33]. Nowadays, DOTA-octapeptides can be used for clinical trials only, and are not commercially available either in the European Union or in the United States.
Since the detection of distant metastasis has a major impact on the choice of treatment, early diagnosis of metastatic spread is crucial. In this regard, it has recently been demonstrated that 68Ga-DOTA-TOC PET is more accurate for detecting bone metastasis (with 97 % sensitivity and 92 % specificity) than CT and conventional bone scintigraphy [34]. A particular indication for 68Ga-peptide PET/CT is for detecting unknown primary NENs (the technique has shown a 59 % detection rate versus 39 % for 111In-pentreteotide, with a subsequent change in patients’ management in about 10 % of cases) [35].
Somatostatin receptor imaging in pulmonary NENs
SRS using 111In-pentetreotide is used mainly in the differentiation of bronchial masses. In fact, 111In-pentetreotide scintigraphy has been shown to be a valuable diagnostic tool for detecting most bronchial carcinoids, including cases associated with ectopic GHRH secretion [36, 37]. In fact, most thoracic NENs exhibit SSTRs, especially sstr2 and sstr5. However, due to the low spatial resolution of the images, SRS has shown a low sensitivity in small tumors. SRS is also helpful in radioguided surgery, making it possible to scan the bed of the tumor after resection and to re-excise an area of increased radioactivity uptake found to correspond to the presence of residual tumor [38]. 68Ga-DOTATOC (-NOC or -TATE) PET/CT has recently been shown to be able to detect lung NENs and to allow better evaluation of the extent of the disease [39]. However, in lung imaging, both SPECT and PET may suffer from respiratory excursions of the thorax; therefore, they show the highest sensitivity for mediastinal lymph node involvement and distant metastases.
Somatostatin receptor imaging in PHEOs/PGLs
Several studies have evaluated the diagnostic accuracy of SRS with 111In-pentreteotide in the evaluation of PHEO and PGL patients. In this regard, it is generally accepted that SRS is able to play a role only in the limited proportion of PHEOs that do not significantly concentrate meta-iodobenzylguanidine (MIBG). SRS is more frequently negative in benign adrenal lesions, because they do not express abundant SSTRs. SRS, therefore, continues to play its main role in the identification of extra-adrenal PGLs or of malignant forms and of their locoregional and distant metastases.
On the other hand, SRS appears to be particularly useful as a functional imaging modality in patients with rapidly progressing and growing PHEOs; in these cases, changes in genetic and cellular characteristics occur, leading to increased expression of SSTRs. Although lymph node, bone, liver and lung metastases can show MIBG uptake, it has been reported that 111In-pentetreotide scintigraphy can identify malignant lesions with a higher degree of accuracy [40]. Furthermore, SRS should be the first choice when a mass is suspected to be PGL on clinical or imaging grounds—this applies particularly to head and neck PGLs—, even though the sensitivity of SRS was recently reported to be less than that of 18F-l-dihydroxyphenylalanine (18F-DOPA) [41]. However, both 68Ga-DOTA-TOC PET/CT and 18F-DOPA PET/CT appear to offer the highest detection rates in non-metastatic extra-adrenal PGLs [42].
Neuroamine uptake imaging
Another characteristic biological feature of certain NENs is their ability to take up amino acids and transform them into biogenic amines through the decarboxylation pathway. Due to this peculiar metabolic pattern, these NENs have, in the past, also been named APUD tumors (the acronym for “Amine Precursor Uptake and Decarboxylation”). NEN cells can thus synthesize catecholamines through an enzymatic pathway, which begins with conversion of the amino acid tyrosine into l-DOPA. l-DOPA is subsequently decarboxylated to dopamine, oxidated to norepinephrine, and methylated to yield epinephrine. These catecholamines are finally transported to and stored in the synaptic vesicles by means of the vesicular transporters for catecholamines (VMAT 1 and VMAT 2). After their release into the synaptic space, re-uptake occurs by norepinephrine transporters located in the presynaptic membrane. Thus, several NENs can be imaged with tracers that target the catecholamine pathway, such as [11C]ephedrine, [11C]hydroxyephedrine, [18F]fluorodopamine (18F-DA), 18F-DOPA and MIBG which is a catecholamine analog taken up by the cells through the norepinephrine transporters and stored in the synaptic vesicles.
MIBG is traditionally the first option for radionuclide imaging of NENs. The diagnostic performance of [123I]MIBG is clearly better than that of [131I]MIBG [43–45], mostly because of the superior imaging quality resulting from the better spatial resolution provided by the 159 keV γ-rays of 123I and the higher signal-to-noise ratio achievable because of the greater injected activity of [123I]MIBG. The higher count rate made possible by administering [123I]MIBG rather than [131I]MIBG also facilitates the performance of SPECT acquisitions. Moreover, physiological uptake of MIBG in normal adrenals is less evident with [123I]MIBG, thus allowing better differentiation of normal condition or compensatory medullary hyperplasia (after unilateral adrenalectomy) from a PHEO [46]. Finally, the patient effective dose in [123I]MIBG scintigraphy is significantly lower than that due to [131I]MIBG. Both [123I]MIBG and [131I]MIBG are approved for clinical diagnostic use in the European Union and the United States.
Literature reports addressing the use of MIBG or 18F-DOPA PET in pulmonary NENs are few and in most cases lung primary tumors are reported in the context of patient populations with NENs of different primary origin.
Neuroamine uptake imaging in PGLs
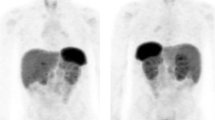
MIBG scintigraphy is frequently used to confirm (with 90 % sensitivity and 95 % specificity) that the detected adrenal mass is indeed a PHEO, while the combined sensitivity of catecholamine measurements and MIBG scintigraphy approaches 100 % [47]. However, in patients without a familial syndrome and with a single unilateral mass and a clear clinical diagnosis, MIBG scintigraphy does not appear to add any information over CT or MRI [48]. Nevertheless, whole-body MIBG scans are particularly helpful in the preoperative identification of multiple primary lesions (Fig. 2) and of metastases from malignant tumors. Functional studies, such as MIBG scintigraphy, are also able to detect small recurrences because the radiotracer accumulates specifically within the tumor and is not affected by postsurgical or postradiotherapy changes.
[123I]MIBG scintigraphy shows the presence of a PHEO in both adrenals and two foci of medullary thyroid carcinoma in a patient with multiple endocrine neoplasia type 2A. Planar images of the thorax and abdomen are shown in a. SPECT/CT slices including thyroid and adrenals are shown in b and c (color figure online)
Several factors can decrease the sensitivity of MIBG scintigraphy. First, some drugs can interfere with the specific MIBG uptake mechanism, thus possibly causing false-negative results; these include both cardiovascular and sympathomimetic drugs (such as anti-arrhythmics for ventricular arrhythmias, combined alpha and beta blockers, adrenergic neuron blockers, alpha blockers, calcium channel blockers, inotropic sympathomimetics, vasoconstrictor sympathomimetics, beta2 stimulants and systemic and local nasal decongestants, and sympathomimetics for glaucoma) and neurological drugs (such as antipsychotics, sedating antihistamines, opioid analgesics, tricyclic anti-depressants, tricyclic-related anti-depressants, and CNS stimulants). Therefore, it is necessary to consider discontinuing, when possible, the administration of these drugs prior to MIBG administration [49].
Suboptimal sensitivity can be due to small size of lesions and/or to their location. In particular, sensitivity can be reduced in the case of extra-adrenal lesions close to sites of physiological uptake/excretion [50]. On the other hand, foci of physiological accumulation of MIBG within the urinary tract or bowel can also be a source of false-positive results. MIBG SPECT evaluated side-by-side with contrast-enhanced CT or MRI and hybrid imaging with SPECT/CT allow more accurate anatomical localization of areas of MIBG uptake, and thus contribute to the interpretation of equivocal findings and the reduction of false-negative and/or false-positive results [51]. Relatively low sensitivity has also been reported in familial syndromes and malignant NENs, suggesting that there is low expression of catecholamine transporters or low affinity of MIBG for these transporters in the familial forms and in poorly differentiated malignant tumors [52].
The sensitivity of MIBG scintigraphy seems to be particularly low for PGLs (17–42 %) especially those located in the head and neck region. While PGLs of the abdomen and thorax can be hormonally functioning, head and neck PGLs are rarely hormonally active, and therefore do not produce excess catecholamines [53–55].
In those circumstances in which the results of MIBG scintigraphy could be affected by low sensitivity (because of lesion site and size, extra-adrenal locations, familial and malignant PHEOs), other radiopharmaceuticals should be considered [55].
The use of PET with 18F-DOPA or 18F-DA is being increasingly reported [56–58], the technique being found to show several advantages over MIBG scintigraphy, in particular higher spatial resolution and low background activity both of which allow the detection of smaller adrenal and extra-adrenal lesions (Fig. 3). Nowadays, 18F-DOPA is commercially available in the European Union, but not in the United States. PET can be performed shortly after the administration of 18F-DOPA or of 18F-DA, as opposed to waiting at least 24 h, as is necessary for MIBG scintigraphy. In addition, thyroid blocking and discontinuation of medications are not necessary for 18F-DOPA PET. Moreover, PET is associated with a lower radiation dose to patients than MIBG scintigraphy [59]. A recent study demonstrated that, while VMAT 1 expression is necessary for MIBG uptake, both VMAT 1 and VMAT 2 seem to be involved in DOPA uptake. Therefore, the presence and function of VMAT 1 and 2 for monoamine transfer into the secretory granules appears to be relevant for the outcome of these techniques [55]. The predominant VMAT 2 expression in parasympathetic paraganglia [55, 60] would explain the high performance of 18F-DOPA PET/CT in head and neck PGLs, which usually derive from parasympathetic ganglia. In the past few years, several studies have highlighted the good diagnostic performance of 18F-DOPA PET/CT [57, 61, 62] for the detection and localization of PHEOs/PGLs. A meta-analysis including 275 patients with suspected PGLs reported the following pooled sensitivities of 18F-DOPA PET/CT in detecting PGLs: 91 % (patient-based) and 79 % (lesion-based) [63]. 18F-DOPA PET/CT has also been recommended in the screening of phenotypically healthy patients carrying SDHx germline mutations, which predispose to the development of NENs [64]. The presence of SDHD mutations seems to be associated with a very high 18F-DOPA patient-based detection rate [55, 61, 62, 65]. Only scarce and uncertain data are available on metastatic and recurrent forms [58, 62].
Neuroamine uptake imaging in GEP NENs
It has been established that SRS is more accurate than MIBG scintigraphy in detecting GEP NENs and their metastases. On the other hand, MIBG scintigraphy can be useful when other methods have failed to depict NENs, or when therapy with [131I]MIBG is being considered [66]. Similar results have been reported for 18F-DOPA PET [67, 68], which is currently recommended only in the case of insulinomas, for preoperative localization in patients with congenital hyperinsulinism. In fact, the ability to take up DOPA and to convert it into dopamine is increased in the hyperfunctional affected pancreatic area in comparison to the normally functioning pancreas, which exhibits low levels of aromatic amino acid decarboxylation. The high sensitivity and exquisite localizing property of 18F-DOPA PET could open up the possibility of performing mini-invasive surgery (in some cases during laparoscopy) and limited resections, thus reducing the risk of long-term diabetes [69]. Conversely, in adult subjects pancreatic uptake of 18F-DOPA is not statistically different between normal subjects and hyperinsulinemic patients, and the main limitation for identifying insulinomas or β-cell hyperplasia is the relatively high uptake of 18F-DOPA in the whole pancreas.
Glucose metabolism imaging
Changes in tumor biology can be characterized by modifications in the expression of receptors or transporters on the cell membrane. Well-differentiated NENs do not overexpress glucose transporters (GLUT), whereas they overexpress SSTRs; poorly differentiated tumors, on the other hand, may show a decline in SSTR density but an increase in GLUT expression.
Thus, evidence has emerged that GEP NENs with a positive [18F]FDG PET have greater aggressiveness irrespective of the classical markers of aggressive pattern, such as grading and Ki67 proliferation index [70]. [18F]FDG PET appears to be a predictor of early tumor progression. [18F]FDG PET and SRS results correlate with progression-free survival and overall survival suggesting that these investigations could be usefully included in the initial workup for metastatic NEN [70]. Therefore, [18F]FDG PET could help in stratifying patients according to their risk and in proper therapy planning [71, 72].
The usefulness of [18F]FDG PET/CT for pulmonary NENs is still debated. In fact, these tumors show variable [18F]FDG uptake, according to mitotic index and tumor proliferation rate. While a low [18F]FDG uptake (SUVmax <2.5) has been observed for typical bronchial carcinoids [73], tumors with a higher grade can show high [18F]FDG uptake. Atypical carcinoids usually appear as small pulmonary nodules with a high SUV on [18F]FDG PET imaging (Fig. 4), sometimes with hilar or mediastinal lymph node metastasis [74]. LCNECs are usually characterized by high [18F]FDG uptake and both PET/CT and stand-alone CT, show high accuracy in disclosing hilar and mediastinal node involvement. However, PET/CT seems to perform better than CT in detecting distant metastases, thus leading to changes in clinical management of patients. It has been reported that an SUVmax greater than 13.7 is associated with short survival, thus suggesting that it may be useful to use [18F]FDG PET/CT not only for staging, but also for prognostic purposes in LCNEC patients [75]. In patients with SCLC, [18F]FDG PET has been reported to be valuable both for initial staging (limited versus extensive spread) and for treatment planning. According to Pandit et al. [76], [18F]FDG PET is also useful for prognostic purposes in SCLC patients, especially treated ones.
[18F]FDG PET/CT. High uptake of the radiopharmaceutical (SUVmax 8.4) in a 1.5 cm nodule within the anterior segment of the upper lobe of the right lung. At pathology the nodule was found to be a G2 neuroendocrine carcinoma (atypical carcinoid) with 8 mitoses per 10 high-power fields (color figure online)
Preliminary data on the combined use of [18F]FDG and 68Ga-DOTA-TOC PET have been reported [74]. Typical carcinoids, that usually overexpress SSTRs, display high 68Ga-DOTA-TOC and low [18F]FDG uptake on PET/CT scan, related to their low proliferative index. It is likely that avidity of [18F]FDG or 68Ga-DOTA-TATE could help to identify, at initial staging (as well as during follow-up and after therapy), aggressive tumors or sites of possible dedifferentiation within indolent tumors.
Although [18F]FDG PET is not recommended as a first-line approach for diagnosing PHEOs, it is of special value for localizing PHEOs when other imaging methods have failed. In fact, some authors reported that most PHEOs accumulate [18F]FDG, especially when malignant. With a sensitivity approaching 100 %, [18F]FDG PET is the preferred functional imaging modality for staging and for treatment monitoring of metastatic PHEOs associated with the SDHB mutation [59].
Targeted radionuclide therapy of NENs
Therapy of NENs is typically multidisciplinary and should be individualized according to the tumor histotype and extension, as well as symptoms. Different new treatments are being suggested and applied by various centers. Nowadays, the therapeutic tools in NENs include surgery, interventional radiology, medical treatments, chemotherapy, new targeted drugs and targeted radionuclide therapy.
Surgery is fundamental in many phases, from the eradication of the primary lesion to the debulking of metastatic lesions, prior to other therapies, to control debilitating symptoms due to hormone overproduction or with a purely palliative intent. The main limitation of surgery is the frequent presence of synchronous metastatic disease.
Interventional radiology techniques are often used in GEP NENs because these tumors commonly spread to the liver. Besides radiofrequency ablation, high-intensity focused ultrasound (HIFU) ablation [77, 78] and radioembolization of liver metastases with yttrium-90 (90Y)-labeled microspheres have been tested in several clinical trials [79, 80].
Medical therapy is aimed at treating symptoms and/or reducing tumor growth. There is little scope for traditional chemotherapy in well-differentiated NENs, since most of these are slow-growing tumors. Multiple single and multi-agent chemotherapy regimens have been utilized, with none showing an objective tumor response greater than 15 % [81]. Cyclophosphamide, vincristine and dacarbazine (CVD) chemotherapy is the first-line regimen most often chosen for the treatment of metastatic and rapidly progressive PHEOs/PGLs, although response rates are suboptimal [82].
Somatostatin analog biotherapy is one of the basic tools for approaching NENs. Somatostatin analogs are generally well tolerated, and long-acting formulations are used successfully to control tumor hypersecretion and symptoms in up to 70 % of patients, early tachyphylaxis is a frequent occurrence [83]. Objective partial responses are encountered in less than 10 % of patients [84]. Nowadays, new molecular targeted drugs (anti-angiogenetic drugs and mTOR inhibitors) are also being explored.
One potential advantage of targeted radionuclide therapy is that it can be administered systemically while minimizing toxicity to non-target healthy tissues. Treatment success depends upon the specificity of the targeting carrier molecule and the choice of an appropriate radioisotope label.
PRRT uses radiolabeled peptides for treating unresectable or metastasized NENs. The rationale for this therapy is to convey radioactivity inside the tumor cells, where sensitive targets, such as DNA, can be hit as a result of internalization of the somatostatin receptor and radiolabeled analog complex.
The tumor candidates for PRRT with radiolabeled somatostatin analogs are, basically, sstr2-expressing NENs, and SRS is currently the most accurate and widely validated method for investigating the presence of SSTR overexpression. Nevertheless, it is likely that 68Ga-DOTA-TOC and 68Ga-DOTA-TATE will become the new standard analogs, since they mimic very closely the in vivo behavior of their 90Y- or Lutetium-177 (177Lu)-labeled counterparts used in PRRT [24].
Several peptides labeled with therapeutic radiometals, such as 90Y or 177Lu, have been explored for therapeutic purposes. 90Y-DOTA-TOC and 177Lu-DOTA-TATE are the most widely employed radiopeptides and can be used in the European Union for therapeutic trials. The choice between these two particular molecules depends on physical and pharmacokinetic considerations. 90Y emits higher energy-range β particles than 177Lu does. The higher energy range of 90Y β particles makes them able to penetrate the tumor more deeply, which is an important physical feature in the case of bulky and/or heterogeneous masses. Moreover, the shorter half-life of 90Y makes it possible to deliver a higher dose rate to the tumor. Instead, the lower energy and range of penetration of the β particles emitted by 177Lu make this molecule more suitable for treating smaller tumors [85]. The pharmacokinetic profiles of both DOTA-TOC and DOTA-TATE are characterized by rapid plasma clearance after administration, with considerable renal excretion. Since the residence times of DOTA-TATE in tumor and kidney are longer than those of DOTA-TOC, 177Lu appears more beneficial for labeling DOTA-TATE, while, in view of the higher renal dose, 90Y appears to be the better radionuclide for labeling DOTA-TOC [86–88]. PRRT, with either 90Y-DOTATOC or 177Lu-DOTATATE, is generally well tolerated, without serious acute side effects. However, the kidneys are the critical organs, particularly after 90Y-DOTA-TOC administration. A median 7.3 % decline in creatinine clearance per year was reported after 90Y-DOTA-TOC therapy, versus 3.8 % per year after 177Lu-DOTA-TATE [89]. The administration of l-lysine and/or l-arginine in order to competitively inhibit the proximal tubular re-absorption of the radiopeptide can reduce the renal dose [85, 90].
The radiopeptide that has been most extensively studied for PRRT is 90Y-DOTA-TOC with a recommended activity per cycle of about 5 GBq (cumulative activities: 13–18.5 GBq) [91]. Despite differences in clinical phase I–II protocols in place at various centers, complete and partial remissions have been recorded in 10–30 % of patients with GEP NENs [92–96]. Toxicity was generally mild and involved the kidneys and bone marrow. True phase II studies with 90Y-DOTA-TOC are still lacking, but experiences in selected series of patients, mostly retrospective, are reported in the literature [97]. A tentative categorization of objective response according to tumor type has been attempted in a meta-analysis of results in GEP tumors. Pancreatic NENs were found to be the tumors responding best to PRRT [98]. Promising clinical results of PRRT with the newer radiopeptide 177Lu-DOTATATE have also been published [99, 100]. More recently, the combination radionuclide therapy using 177Lu- and 90Y-labeled radiopeptides was proposed to improve efficacy in patients with tumors of various sizes and non-homogeneous receptor distribution [101–103].
Targeted radionuclide therapy with [131I]MIBG has been evaluated in several NENs. While [131I]MIBG is approved for treatment in the European Union, it is still an investigational new drug (IND) in the United States, where it can be used for therapy trials in accordance with USP regulations. The foundations of this treatment approach are: the adequate cellular uptake and retention of MIBG and the emission from 131I of low energy β particles (0.61 MeV max), as well as the principal gamma ray of 364 keV, with a physical half-life of 8.04 days. Unresectable PHEOs, PGLs or carcinoids, advanced neuroblastomas, and metastatic medullary thyroid carcinomas are the tumors most frequently treated with [131I]MIBG.
Prior to [131I]MIBG therapy, candidates for the treatment have to undergo a diagnostic MIBG study to ensure that adequate MIBG targeting is possible. Different criteria based on tumor uptake have been proposed to classify a patient as eligible for MIBG treatment [104–107].
Patient preparation includes discontinuation of drugs interfering with MIBG uptake, such as labetalol, tricyclic antidepressants, reserpine, and some sympathomimetics [108]. Thyroid blocking using potassium iodide (KI) or lugol solution, also in combination with potassium perchlorate, is necessary, generally starting 1 or 2 days prior to treatment and continuing for 14 days after treatment [108].
Slow administration of [131I]MIBG—the suggested administration duration ranges from 45 min to 4 h [108]—is mandatory to minimize the possible pharmacological, mass-related effects of MIBG. For the same reason, the specific activity of [131I]MIBG used for therapeutic purposes should be higher than that used for diagnostic purposes (up to 1.48 GBq/mg) [108] to reduce the incidence of pharmacological effects of the carrier MIBG, such as nausea, vomiting and hypertension [109].
Although [131I]MIBG therapy has its best defined role in patients with PHEOs/PGLs, published data are limited and there exist few prospective trials. In these patients symptomatic and biochemical improvements are frequently observed after treatment, while complete response rates are low. In a meta-analysis of 116 patients treated with [131I]MIBG (mean single activity of 158 mCi, mean cumulative activity 490 mCi), 4 % had a complete response, 26 % a partial response, 57 % stable disease, and 13 % disease progression. Biochemical catecholamine response was demonstrated in 55 % of the patients [110].
There is currently no consensus on the optimal dosing strategy in radionuclide therapy of NENs. In patients treated with median activities of 268 versus 149 mCi, higher activities seemed to deliver the desired dose faster with a modest increase in toxicity, but with similar overall response rates [111].
Since somatostatin analogs exhibit better targeting properties, the role of [131I]MIBG therapy in patients with GEP NENs is limited to SRS-negative patients, to those with borderline renal function, and to patients resistant to somatostatin therapy, as a palliative option.
In neuroblastoma, most studies have used [131I]MIBG with therapeutic intent, and good responses have been obtained in selected patients with advanced disease in whom first-line therapy failed. The response rates in these studies using single doses or large cumulative doses of [131I]MIBG ranged from 25 to 46 % [112–115]. The actual benefit of multiple treatments is still debated.
Conclusions
Because of their heterogeneity in terms of receptor expression and/or metabolic processes, NENs can be imaged and treated with multitracer approaches that target various biological properties of the cells from which the tumor originates. Radiopeptides with high affinity for different SSTRs as well as radiopharmaceuticals involved in various steps of the neuroamine uptake process or in glucose metabolism are becoming increasingly widely available. For GEP NENs, SRS remains the investigation of choice, although several studies have shown PET with 68Ga-DOTA-peptides to have the better diagnostic accuracy. The fact that these radiopharmaceuticals have not yet been authorized/approved for clinical use is one of the factors preventing their diffusion on a wide scale. MIBG scintigraphy and 18F-DOPA PET are generally a second option in the evaluation of GEP NENs. 18F-DOPA, in particular, is employed for imaging patients with hyperinsulinism. SRS is also the first-line investigation in lung NENs. Instead, in poorly differentiated, more aggressive NENs, [18F]FDG PET plays a predominant role, while in PHEOs/PGLs, MIBG scintigraphy is generally the examination of choice. However, the superior resolution of PET has led to an increasingly frequent use of 18F-DOPA, which is commercially available in the European Union. Moreover, 18F-DOPA is much more sensitive in the diagnosis of head and neck PGLs, representing, together with PET with DOTA-peptides, the best diagnostic approach in this field. The identification of new radiotracers with higher specificity will constitute the basis for new diagnostic imaging approaches in NENs.
Targeted radionuclide therapy with either [131I]MIBG or PRRT constitutes an important therapeutic option in patients with unresectable or metastasized NENs, although more prospective-controlled studies are desirable to better define its efficacy. The use of targeted radionuclide therapy in combination with chemotherapy agents will emerge as one of the main challenges in the near future.
References
Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S (2010) The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 39:707–712
Patel YC (1999) Somatostatin and its receptor family. Front Neuroendocrinol 20:157–198
Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP, Moss SF, Nilsson O, Rindi G, Salazar R, Ruszniewski P, Sundin A (2008) Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 9:61–72
Alexandraki KI, Grossman AB (2010) The ectopic ACTH syndrome. Rev Endocr Metab Disord 11:117–126
Borson-Chazot F, Garby L, Raverot G, Claustrat F, Raverot V, Sassolas G, GTE group (2012) Acromegaly induced by ectopic secretion of GHRH: a review 30 years after GHRH discovery. Ann Endocrinol (Paris) 73:497–502
Daddi N, Urbani M, Semeraro A, Capozzi R, Scarpelli G, Avenia N, Puma F, Ferolla P, Ribacchi R, Daddi G (2008) Surgical treatment of well differentiated neuroendocrine tumours of the lung. G Chir 29:246–249
Ferolla P, Faggiano A, Avenia N, Milone F, Masone S, Giampaglia F, Puma F, Daddi G, Angeletti G, Lombardi G, Santeusanio F, Colao A (2007) Epidemiology of non-gastroenteropancreatic (neuro)endocrine tumours. Clin Endocrinol (Oxf) 66:1–6
Fink G, Krelbaum T, Yellin A, Bendayan D, Saute M, Glazer M, Kramer MR (2001) Pulmonary carcinoid: presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest 119:1647–1651
Ilias I, Pacak K (2008) A clinical overview of pheochromocytomas/paragangliomas and carcinoid tumors. Nucl Med Biol 35(Suppl 1):S27–S34
Lenders JW, Eisenhofer G, Mannelli M, Pacak K (2005) Phaeochromocytoma. Lancet 366:665–675
Mankoff DA, Eary JF, Link JM, Muzi M, Rajendran JG, Spence AM, Krohn KA (2007) Tumor-specific positron emission tomography imaging in patients: [18F]fluorodeoxyglucose and beyond. Clin Cancer Res 13:3460–3469
Lewington VJ, Clarke SE (2001) Isotopic evaluation and therapy in patients with malignant endocrine disease. Best Pract Res Clin Endocrinol Metab 15:225–239
Wong F, Kim E (2001) Peptide receptor imaging. In: Kim E, Yang D (eds) Targeted molecular imaging in oncology. Springer, New York, pp 102–110
Kwekkeboom DJ, Krenning EP, Scheidhauer K, Lewington V, Lebtahi R, Grossman A, Vitek P, Sundin A, Plockinger U, Mallorca Consensus Conference participants, European Neuroendocrine Tumor Society (2009) ENETS consensus guidelines for the standards of care in neuroendocrine tumors: somatostatin receptor imaging with 111In-pentetreotide. Neuroendocrinology 90:184–189
Signore A, Procaccini E, Chianelli M, Salerno G, Iozzo P, Annovazzi A, Leonetti F, Tamburrano G, Ronga G (1995) SPECT imaging with 111In-octreotide for the localization of pancreatic insulinoma. Q J Nucl Med 39(4 Suppl 1):111–112
Hillel PG, van Beek EJ, Taylor C, Lorenz E, Bax ND, Prakash V, Tindale WB (2006) The clinical impact of a combined gamma camera/CT imaging system on somatostatin receptor imaging of neuroendocrine tumours. Clin Radiol 61:579–587
Schillaci O, Spanu A, Scopinaro F, Falchi A, Danieli R, Marongiu P, Pisu N, Madeddu G, Delle Fave G, Madeddu G (2003) Somatostatin receptor scintigraphy in liver metastasis detection from gastroenteropancreatic neuroendocrine tumors. J Nucl Med 44:359–368
Perri M, Erba P, Volterrani D, Lazzeri E, Boni G, Grosso M, Mariani G (2008) Octreo-SPECT/CT imaging for accurate detection and localization of suspected neuroendocrine tumors. Q J Nucl Med Mol Imaging 52:323–333
Gibril F, Jensen RT (2004) Diagnostic uses of radiolabelled somatostatin receptor analogues in gastroenteropancreatic endocrine tumours. Dig Liver Dis 36(Suppl 1):S106–S120
Krenning EP, Kwekkeboom DJ, Bakker WH, Breeman WA, Kooij PP, Oei HY, van Hagen M, Postema PT, de Jong M, Reubi JC et al (1993) Somatostatin receptor scintigraphy with [111In-DTPA-d-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med 20:716–731
Jamar F, Fiasse R, Leners N, Pauwels S (1995) Somatostatin receptor imaging with indium-111-pentetreotide in gastroenteropancreatic neuroendocrine tumors: safety, efficacy and impact on patient management. J Nucl Med 36:542–549
Kaltsas G, Rockall A, Papadogias D, Reznek R, Grossman AB (2004) Recent advances in radiological and radionuclide imaging and therapy of neuroendocrine tumours. Eur J Endocrinol 151:15–27
Kwekkeboom D, Krenning EP, de Jong M (2000) Peptide receptor imaging and therapy. J Nucl Med 41:1704–1713
Kwekkeboom DJ, Kam BL, van Essen M, Teunissen JJ, van Eijck CH, Valkema R, de Jong M, de Herder WW, Krenning EP (2010) Somatostatin receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer 17:R53–R73
Gunn SH, Schwimer JE, Cox M, Anthony CT, O’Dorisio MS, Woltering EA (2006) In vitro modeling of the clinical interactions between octreotide and 111In-pentetreotide: is there evidence of somatostatin receptor downregulation? J Nucl Med 47:354–359
Gopinath G, Ahmed A, Buscombe JR, Dickson JC, Caplin ME, Hilson AJ (2004) Prediction of clinical outcome in treated neuroendocrine tumours of carcinoid type using functional volumes on 111In-pentetreotide SPECT imaging. Nucl Med Commun 25:253–257
Buchmann I, Henze M, Engelbrecht S, Eisenhut M, Runz A, Schafer M, Schilling T, Haufe S, Herrmann T, Haberkorn U (2007) Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging 34:1617–1626
Junik R, Drobik P, Malkowski B, Kobus-Blachnio K (2006) The role of positron emission tomography (PET) in diagnostics of gastroenteropancreatic neuroendocrine tumours (GEP NET). Adv Med Sci 51:66–68
Antunes P, Ginj M, Zhang H, Waser B, Baum RP, Reubi JC, Maecke H (2007) Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging 34:982–993
Kabasakal L, Demirci E, Ocak M, Decristoforo C, Araman A, Ozsoy Y, Uslu I, Kanmaz B (2012) Comparison of 68Ga-DOTATATE and 68Ga-DOTANOC PET/CT imaging in the same patient group with neuroendocrine tumours. Eur J Nucl Med Mol Imaging 39:1271–1277
Poeppel TD, Binse I, Petersenn S, Lahner H, Schott M, Antoch G, Brandau W, Bockisch A, Boy C (2013) Differential uptake of 68Ga-DOTATOC and 68Ga-DOTATATE in PET/CT of gastroenteropancreatic neuroendocrine tumors. Recent Results Cancer Res 194:353–371
Poeppel TD, Binse I, Petersenn S, Lahner H, Schott M, Antoch G, Brandau W, Bockisch A, Boy C (2011) 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J Nucl Med 52:1864–1870
Wild D, Bomanji JB, Benkert P, Maecke H, Ell PJ, Reubi JC, Caplin ME (2013) Comparison of 68Ga-DOTANOC and 68Ga-DOTATATE PET/CT within patients with gastroenteropancreatic neuroendocrine tumors. J Nucl Med 54:364–372
Putzer D, Gabriel M, Henninger B, Kendler D, Uprimny C, Dobrozemsky G, Decristoforo C, Bale RJ, Jaschke W, Virgolini IJ (2009) Bone metastases in patients with neuroendocrine tumor: 68Ga-DOTA-Tyr3-octreotide PET in comparison to CT and bone scintigraphy. J Nucl Med 50:1214–1221
Prasad V, Ambrosini V, Hommann M, Hoersch D, Fanti S, Baum RP (2010) Detection of unknown primary neuroendocrine tumours (CUP-NET) using 68Ga-DOTA-NOC receptor PET/CT. Eur J Nucl Med Mol Imaging 37:67–77
Krenning EP, Kwekkeboom DJ, Reubi JC, Van Hagen PM, van Eijck CH, Oei HY, Lamberts SW (1992) 111In-octreotide scintigraphy in oncology. Metabolism 41:83–86
Tsagarakis S, Christoforaki M, Giannopoulou H, Rondogianni F, Housianakou I, Malagari C, Rontogianni D, Bellenis I, Thalassinos N (2003) A reappraisal of the utility of somatostatin receptor scintigraphy in patients with ectopic adrenocorticotropin Cushing’s syndrome. J Clin Endocrinol Metab 88:4754–4758
Rodriguez JA, Meyers MO, Jacome TH, Failla P, Harrison LH Jr (2002) Intraoperative detection of a bronchial carcinoid with a radiolabeled somatostatin analog. Chest 121:985–988
Ambrosini V, Castellucci P, Rubello D, Nanni C, Musto A, Allegri V, Montini GC, Mattioli S, Grassetto G, Al-Nahhas A, Franchi R, Fanti S (2009) 68Ga-DOTA-NOC: a new PET tracer for evaluating patients with bronchial carcinoid. Nucl Med Commun 30:281–286
Ilias I, Chen CC, Carrasquillo JA, Whatley M, Ling A, Lazurova I, Adams KT, Perera S, Pacak K (2008) Comparison of 6-18F-fluorodopamine PET with 123I-metaiodobenzylguanidine and 111In-pentetreotide scintigraphy in localization of nonmetastatic and metastatic pheochromocytoma. J Nucl Med 49:1613–1619
King KS, Chen CC, Alexopoulos DK, Whatley MA, Reynolds JC, Patronas N, Ling A, Adams KT, Xekouki P, Lando H, Stratakis CA, Pacak K (2011) Functional imaging of SDHx-related head and neck paragangliomas: comparison of 18F-fluorodihydroxyphenylalanine, 18F-fluorodopamine, 18F-fluoro-2-deoxy-d-glucose PET, 123I-metaiodobenzylguanidine scintigraphy, and 111In-pentetreotide scintigraphy. J Clin Endocrinol Metab 96:2779–2785
Kroiss A, Putzer D, Frech A, Decristoforo C, Uprimny C, Gasser RW, Shulkin BL, Url C, Widmann G, Prommegger R, Sprinzl GM, Fraedrich G, Virgolini IJ (2013) A retrospective comparison between Ga-DOTA-TOC PET/CT and F-DOPA PET/CT in patients with extra-adrenal paraganglioma. Eur J Nucl Med Mol Imaging 40:1800–1808
Cecchin D, Lumachi F, Marzola MC, Opocher G, Scaroni C, Zucchetta P, Mantero F, Bui F (2006) A meta-iodobenzylguanidine scintigraphic scoring system increases accuracy in the diagnostic management of pheochromocytoma. Endocr Relat Cancer 13:525–533
Furuta N, Kiyota H, Yoshigoe F, Hasegawa N, Ohishi Y (1999) Diagnosis of pheochromocytoma using [123I]-compared with [131I]-metaiodobenzylguanidine scintigraphy. Int J Urol 6:119–124
Nakatani T, Hayama T, Uchida J, Nakamura K, Takemoto Y, Sugimura K (2002) Diagnostic localization of extra-adrenal pheochromocytoma: comparison of 123I-MIBG imaging and 131I-MIBG imaging. Oncol Rep 9:1225–1227
Lynn MD, Shapiro B, Sisson JC, Beierwaltes WH, Meyers LJ, Ackerman R, Mangner TJ (1985) Pheochromocytoma and the normal adrenal medulla: improved visualization with I-123 MIBG scintigraphy. Radiology 155:789–792
Guller U, Turek J, Eubanks S, Delong ER, Oertli D, Feldman JM (2006) Detecting pheochromocytoma: defining the most sensitive test. Ann Surg 243:102–107
Miskulin J, Shulkin BL, Doherty GM, Sisson JC, Burney RE, Gauger PG (2003) Is preoperative iodine 123 meta-iodobenzylguanidine scintigraphy routinely necessary before initial adrenalectomy for pheochromocytoma? Surgery 134:918–922 (discussion 922–923)
Bombardieri E, Giammarile F, Aktolun C, Baum RP, Bischof Delaloye A, Maffioli L, Moncayo R, Mortelmans L, Pepe G, Reske SN, Castellani MR, Chiti A, European Association for Nuclear Medicine (2010) 131I/123I-metaiodobenzylguanidine (mIBG) scintigraphy: procedure guidelines for tumour imaging. Eur J Nucl Med Mol Imaging 37:2436–2446
Bhatia KS, Ismail MM, Sahdev A, Rockall AG, Hogarth K, Canizales A, Avril N, Monson JP, Grossman AB, Reznek RH (2008) 123I-metaiodobenzylguanidine (MIBG) scintigraphy for the detection of adrenal and extra-adrenal phaeochromocytomas: CT and MRI correlation. Clin Endocrinol (Oxf) 69:181–188
Rozovsky K, Koplewitz BZ, Krausz Y, Revel-Vilk S, Weintraub M, Chisin R, Klein M (2008) Added value of SPECT/CT for correlation of MIBG scintigraphy and diagnostic CT in neuroblastoma and pheochromocytoma. AJR Am J Roentgenol 190:1085–1090
Kaji P, Carrasquillo JA, Linehan WM, Chen CC, Eisenhofer G, Pinto PA, Lai EW, Pacak K (2007) The role of 6-[18F]fluorodopamine positron emission tomography in the localization of adrenal pheochromocytoma associated with von Hippel-Lindau syndrome. Eur J Endocrinol 156:483–487
Koopmans KP, Jager PL, Kema IP, Kerstens MN, Albers F, Dullaart RP (2008) 111In-octreotide is superior to 123I-metaiodobenzylguanidine for scintigraphic detection of head and neck paragangliomas. J Nucl Med 49:1232–1237
Milardovic R, Corssmit EP, Stokkel M (2010) Value of 123I-MIBG scintigraphy in paraganglioma. Neuroendocrinology 91:94–100
Fottner C, Helisch A, Anlauf M, Rossmann H, Musholt TJ, Kreft A, Schadmand-Fischer S, Bartenstein P, Lackner KJ, Kloppel G, Schreckenberger M, Weber MM (2010) 6-18F-fluoro-l-dihydroxyphenylalanine positron emission tomography is superior to 123I-metaiodobenzyl-guanidine scintigraphy in the detection of extraadrenal and hereditary pheochromocytomas and paragangliomas: correlation with vesicular monoamine transporter expression. J Clin Endocrinol Metab 95:2800–2810
Fiebrich HB, Brouwers AH, Kerstens MN, Pijl ME, Kema IP, de Jong JR, Jager PL, Elsinga PH, Dierckx RA, van der Wal JE, Sluiter WJ, de Vries EG, Links TP (2009) 6-[F-18]Fluoro-l-dihydroxyphenylalanine positron emission tomography is superior to conventional imaging with 123I-metaiodobenzylguanidine scintigraphy, computer tomography, and magnetic resonance imaging in localizing tumors causing catecholamine excess. J Clin Endocrinol Metab 94:3922–3930
Hoegerle S, Nitzsche E, Altehoefer C, Ghanem N, Manz T, Brink I, Reincke M, Moser E, Neumann HP (2002) Pheochromocytomas: detection with 18F DOPA whole body PET-Initial results. Radiology 222:507–512
Rufini V, Treglia G, Castaldi P, Perotti G, Calcagni ML, Corsello SM, Galli G, Fanti S, Giordano A (2011) Comparison of 123I-MIBG SPECT-CT and 18F-DOPA PET-CT in the evaluation of patients with known or suspected recurrent paraganglioma. Nucl Med Commun 32:575–582
Pacak K, Eisenhofer G, Ilias I (2009) Diagnosis of pheochromocytoma with special emphasis on MEN2 syndrome. Hormones (Athens) 8:111–116
Peter D, Liu Y, Sternini C, de Giorgio R, Brecha N, Edwards RH (1995) Differential expression of two vesicular monoamine transporters. J Neurosci 15:6179–6188
Rischke HC, Benz MR, Wild D, Mix M, Dumont RA, Campbell D, Seufert J, Wiech T, Rossler J, Weber WA, Neumann HP (2012) Correlation of the genotype of paragangliomas and pheochromocytomas with their metabolic phenotype on 3,4-dihydroxy-6-18F-fluoro-l-phenylalanin PET. J Nucl Med 53:1352–1358
Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, Eisenhofer G, Martiniova L, Adams KT, Pacak K (2009) Comparison of 18F-fluoro-l-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab 94:4757–4767
Treglia G, Cocciolillo F, de Waure C, Di Nardo F, Gualano MR, Castaldi P, Rufini V, Giordano A (2012) Diagnostic performance of 18F-dihydroxyphenylalanine positron emission tomography in patients with paraganglioma: a meta-analysis. Eur J Nucl Med Mol Imaging 39:1144–1153
Miederer M, Fottner C, Rossmann H, Helisch A, Papaspyrou K, Bartsch O, Mann WJ, Musholt TJ, Weber MM, Lackner KJ, Schreckenberger M (2013) High incidence of extraadrenal paraganglioma in families with SDHx syndromes detected by functional imaging with [18F]fluorodihydroxyphenylalanine PET. Eur J Nucl Med Mol Imaging 40:889–896
Marzola MC, Chondrogiannis S, Grassetto G, Rampin L, Maffione AM, Ferretti A, Opocher G, Schiavi F, Colletti PM, Rubello D (2014) 18F-DOPA PET/CT in the evaluation of hereditary SDH-deficiency paraganglioma-pheochromocytoma syndromes. Clin Nucl Med 39:e53–e58. doi:10.1097/RLU.0b013e31829aface
Ezziddin S, Logvinski T, Yong-Hing C, Ahmadzadehfar H, Fischer HP, Palmedo H, Bucerius J, Reinhardt MJ, Biersack HJ (2006) Factors predicting tracer uptake in somatostatin receptor and MIBG scintigraphy of metastatic gastroenteropancreatic neuroendocrine tumors. J Nucl Med 47:223–233
Ambrosini V, Tomassetti P, Castellucci P, Campana D, Montini G, Rubello D, Nanni C, Rizzello A, Franchi R, Fanti S (2008) Comparison between 68Ga-DOTA-NOC and 18F-DOPA PET for the detection of gastro-entero-pancreatic and lung neuro-endocrine tumours. Eur J Nucl Med Mol Imaging 35:1431–1438
Haug A, Auernhammer CJ, Wangler B, Tiling R, Schmidt G, Goke B, Bartenstein P, Pöpperl G (2009) Intraindividual comparison of 68Ga-DOTA-TATE and 18F-DOPA PET in patients with well-differentiated metastatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging 36:765–770
Mohnike K, Blankenstein O, Minn H, Mohnike W, Fuchtner F, Otonkoski T (2008) [18F]-DOPA positron emission tomography for preoperative localization in congenital hyperinsulinism. Horm Res 70:65–72
Garin E, Le Jeune F, Devillers A, Cuggia M, de Lajarte-Thirouard AS, Bouriel C, Boucher E, Raoul JL (2009) Predictive value of 18F-FDG PET and somatostatin receptor scintigraphy in patients with metastatic endocrine tumors. J Nucl Med 50:858–864
Oh S, Prasad V, Lee DS, Baum RP (2011) Effect of peptide receptor radionuclide therapy on somatostatin receptor status and glucose metabolism in neuroendocrine tumors: intraindividual comparison of Ga-68 DOTANOC PET/CT and F-18 FDG PET/CT. Int J Mol Imaging 2011:524130
Severi S, Nanni O, Bodei L, Sansovini M, Ianniello A, Nicoletti S, Scarpi E, Matteucci F, Gilardi L, Paganelli G (2013) Role of 18FDG PET/CT in patients treated with 177Lu-DOTATATE for advanced differentiated neuroendocrine tumours. Eur J Nucl Med Mol Imaging 40:881–888
Erasmus JJ, McAdams HP, Patz EF Jr, Coleman RE, Ahuja V, Goodman PC (1998) Evaluation of primary pulmonary carcinoid tumors using FDG PET. AJR Am J Roentgenol 170:1369–1373
Kayani I, Conry BG, Groves AM, Win T, Dickson J, Caplin M, Bomanji JB (2009) A comparison of 68Ga-DOTATATE and 18F-FDG PET/CT in pulmonary neuroendocrine tumors. J Nucl Med 50:1927–1932
Chong S, Lee KS, Chung MJ, Han J, Kwon OJ, Kim TS (2006) Neuroendocrine tumors of the lung: clinical, pathologic, and imaging findings. Radiographics 26:41–57 (discussion 57–48)
Pandit N, Gonen M, Krug L, Larson SM (2003) Prognostic value of [18F]FDG-PET imaging in small cell lung cancer. Eur J Nucl Med Mol Imaging 30:78–84
Dubinsky TJ, Cuevas C, Dighe MK, Kolokythas O, Hwang JH (2008) High-intensity focused ultrasound: current potential and oncologic applications. AJR Am J Roentgenol 190:191–199
Mazzaglia PJ, Berber E, Siperstein AE (2007) Radiofrequency thermal ablation of metastatic neuroendocrine tumors in the liver. Curr Treat Options Oncol 8:322–330
Kennedy AS, Dezarn WA, McNeillie P, Coldwell D, Nutting C, Carter D, Murthy R, Rose S, Warner RR, Liu D, Palmedo H, Overton C, Jones B, Salem R (2008) Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol 31:271–279
O’Toole D, Ruszniewski P (2005) Chemoembolization and other ablative therapies for liver metastases of gastrointestinal endocrine tumours. Best Pract Res Clin Gastroenterol 19:585–594
Modlin IM, Latich I, Kidd M, Zikusoka M, Eick G (2006) Therapeutic options for gastrointestinal carcinoids. Clin Gastroenterol Hepatol 4:526–547
Huang H, Abraham J, Hung E, Averbuch S, Merino M, Steinberg SM, Pacak K, Fojo T (2008) Treatment of malignant pheochromocytoma/paraganglioma with cyclophosphamide, vincristine, and dacarbazine: recommendation from a 22-year follow-up of 18 patients. Cancer 113:2020–2028
Hofland LJ, Lamberts SW (2003) The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr Rev 24:28–47
Saltz L, Trochanowski B, Buckley M, Heffernan B, Niedzwiecki D, Tao Y, Kelsen D (1993) Octreotide as an antineoplastic agent in the treatment of functional and nonfunctional neuroendocrine tumors. Cancer 72:244–248
Brans B, Bodei L, Giammarile F, Linden O, Luster M, Oyen WJ, Tennvall J (2007) Clinical radionuclide therapy dosimetry: the quest for the “Holy Gray”. Eur J Nucl Med Mol Imaging 34:772–786
Esser JP, Krenning EP, Teunissen JJ, Kooij PP, van Gameren AL, Bakker WH, Kwekkeboom DJ (2006) Comparison of [177Lu-DOTA0, Tyr3]octreotate and [177Lu-DOTA0, Tyr3]octreotide: which peptide is preferable for PRRT? Eur J Nucl Med Mol Imaging 33:1346–1351
Virgolini I, Britton K, Buscombe J, Moncayo R, Paganelli G, Riva P (2002) In- and Y-DOTA-lanreotide: results and implications of the MAURITIUS trial. Semin Nucl Med 32:148–155
Wehrmann C, Senftleben S, Zachert C, Muller D, Baum RP (2007) Results of individual patient dosimetry in peptide receptor radionuclide therapy with 177Lu DOTA-TATE and 177Lu DOTA-NOC. Cancer Biother Radiopharm 22:406–416
Bodei L, Cremonesi M, Ferrari M, Pacifici M, Grana CM, Bartolomei M, Baio SM, Sansovini M, Paganelli G (2008) Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. Eur J Nucl Med Mol Imaging 35:1847–1856
Bernard BF, Krenning EP, Breeman WA, Rolleman EJ, Bakker WH, Visser TJ, Macke H, de Jong M (1997) d-lysine reduction of indium-111 octreotide and yttrium-90 octreotide renal uptake. J Nucl Med 38:1929–1933
Bodei L, Cremonesi M, Zoboli S, Grana C, Bartolomei M, Rocca P, Caracciolo M, Macke HR, Chinol M, Paganelli G (2003) Receptor-mediated radionuclide therapy with 90Y-DOTATOC in association with amino acid infusion: a phase I study. Eur J Nucl Med Mol Imaging 30:207–216
Otte A, Herrmann R, Heppeler A, Behe M, Jermann E, Powell P, Maecke HR, Muller J (1999) Yttrium-90 DOTATOC: first clinical results. Eur J Nucl Med 26:1439–1447
Paganelli G, Zoboli S, Cremonesi M, Bodei L, Ferrari M, Grana C, Bartolomei M, Orsi F, De Cicco C, Macke HR, Chinol M, de Braud F (2001) Receptor-mediated radiotherapy with 90Y-DOTA-d-Phe1-Tyr3-octreotide. Eur J Nucl Med 28:426–434
Waldherr C, Pless M, Maecke HR, Schumacher T, Crazzolara A, Nitzsche EU, Haldemann A, Mueller-Brand J (2002) Tumor response and clinical benefit in neuroendocrine tumors after 7.4 GBq 90Y-DOTATOC. J Nucl Med 43:610–616
Valkema R, De Jong M, Bakker WH, Breeman WA, Kooij PP, Lugtenburg PJ, De Jong FH, Christiansen A, Kam BL, De Herder WW, Stridsberg M, Lindemans J, Ensing G, Krenning EP (2002) Phase I study of peptide receptor radionuclide therapy with [In-DTPA]octreotide: the Rotterdam experience. Semin Nucl Med 32:110–122
Valkema R, Pauwels S, Kvols LK, Barone R, Jamar F, Bakker WH, Kwekkeboom DJ, Bouterfa H, Krenning EP (2006) Survival and response after peptide receptor radionuclide therapy with [90Y-DOTA0, Tyr3]octreotide in patients with advanced gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med 36:147–156
Bodei L, Cremonesi M, Grana C, Rocca P, Bartolomei M, Chinol M, Paganelli G (2004) Receptor radionuclide therapy with 90Y-[DOTA]0-Tyr3-octreotide (90Y-DOTATOC) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging 31:1038–1046
Plockinger U, Rindi G, Arnold R, Eriksson B, Krenning EP, de Herder WW, Goede A, Caplin M, Oberg K, Reubi JC, Nilsson O, Delle Fave G, Ruszniewski P, Ahlman H, Wiedenmann B, European Neuroendocrine Tumour Society (2004) Guidelines for the diagnosis and treatment of neuroendocrine gastrointestinal tumours. A consensus statement on behalf of the European neuroendocrine tumour society (ENETS). Neuroendocrinology 80:394–424
Kwekkeboom DJ, Teunissen JJ, Bakker WH, Kooij PP, de Herder WW, Feelders RA, van Eijck CH, Esser JP, Kam BL, Krenning EP (2005) Radiolabeled somatostatin analog [177Lu-DOTA0, Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol 23:2754–2762
Teunissen JJ, Kwekkeboom DJ, Krenning EP (2004) Quality of life in patients with gastroenteropancreatic tumors treated with [177Lu-DOTA0, Tyr3]octreotate. J Clin Oncol 22:2724–2729
de Jong M, Breeman WA, Valkema R, Bernard BF, Krenning EP (2005) Combination radionuclide therapy using 177Lu- and 90Y-labeled somatostatin analogs. J Nucl Med 46(Suppl 1):13S–17S
Kunikowska J, Krolicki L, Hubalewska-Dydejczyk A, Mikolajczak R, Sowa-Staszczak A, Pawlak D (2011) Clinical results of radionuclide therapy of neuroendocrine tumours with 90Y-DOTATATE and tandem 90Y/177Lu-DOTATATE: which is a better therapy option? Eur J Nucl Med Mol Imaging 38:1788–1797
Seregni E, Maccauro M, Coliva A, Castellani MR, Bajetta E, Aliberti G, Vellani C, Chiesa C, Martinetti A, Bogni A, Bombardieri E (2010) Treatment with tandem [90Y]DOTA-TATE and [177Lu] DOTA-TATE of neuroendocrine tumors refractory to conventional therapy: preliminary results. Q J Nucl Med Mol Imaging 54:84–91
Gonias S, Goldsby R, Matthay KK, Hawkins R, Price D, Huberty J, Damon L, Linker C, Sznewajs A, Shiboski S, Fitzgerald P (2009) Phase II study of high-dose [131I]metaiodobenzylguanidine therapy for patients with metastatic pheochromocytoma and paraganglioma. J Clin Oncol 27:4162–4168
Krempf M, Lumbroso J, Mornex R, Brendel AJ, Wemeau JL, Delisle MJ, Aubert B, Carpentier P, Fleury-Goyon MC, Gibold C et al (1991) Use of m-[131I]iodobenzylguanidine in the treatment of malignant pheochromocytoma. J Clin Endocrinol Metab 72:455–461
Mukherjee JJ, Kaltsas GA, Islam N, Plowman PN, Foley R, Hikmat J, Britton KE, Jenkins PJ, Chew SL, Monson JP, Besser GM, Grossman AB (2001) Treatment of metastatic carcinoid tumours, phaeochromocytoma, paraganglioma and medullary carcinoma of the thyroid with 131I-meta-iodobenzylguanidine [131I-mIBG]. Clin Endocrinol (Oxf) 55:47–60
Shapiro B, Sisson JC, Lloyd R, Nakajo M, Satterlee W, Beierwaltes WH (1984) Malignant phaeochromocytoma: clinical, biochemical and scintigraphic characterization. Clin Endocrinol (Oxf) 20:189–203
Giammarile F, Chiti A, Lassmann M, Brans B, Flux G, EANM (2008) EANM procedure guidelines for 131I-meta-iodobenzylguanidine (131I-mIBG) therapy. Eur J Nucl Med Mol Imaging 35:1039–1047
Barrett JA, Joyal JL, Hillier SM, Maresca KP, Femia FJ, Kronauge JF, Boyd M, Mairs RJ, Babich JW (2010) Comparison of high-specific-activity ultratrace 123/131I-MIBG and carrier-added 123/131I-MIBG on efficacy, pharmacokinetics, and tissue distribution. Cancer Biother Radiopharm 25:299–308
Loh KC, Fitzgerald PA, Matthay KK, Yeo PP, Price DC (1997) The treatment of malignant pheochromocytoma with iodine-131 metaiodobenzylguanidine (131I-MIBG): a comprehensive review of 116 reported patients. J Endocrinol Invest 20:648–658
Castellani MR, Seghezzi S, Chiesa C, Aliberti GL, Maccauro M, Seregni E, Orunesu E, Luksch R, Bombardieri E (2010) 131I-MIBG treatment of pheochromocytoma: low versus intermediate activity regimens of therapy. Q J Nucl Med Mol Imaging 54:100–113
Hoefnagel CA, Voute PA, De Kraker J, Valdes Olmos RA (1991) [131I]metaiodobenzylguanidine therapy after conventional therapy for neuroblastoma. J Nucl Biol Med 35:202–206
Howard JP, Maris JM, Kersun LS, Huberty JP, Cheng SC, Hawkins RA, Matthay KK (2005) Tumor response and toxicity with multiple infusions of high dose 131I-MIBG for refractory neuroblastoma. Pediatr Blood Cancer 44:232–239
Lashford LS, Lewis IJ, Fielding SL, Flower MA, Meller S, Kemshead JT, Ackery D (1992) Phase I/II study of iodine 131 metaiodobenzylguanidine in chemoresistant neuroblastoma: a United Kingdom children’s cancer study group investigation. J Clin Oncol 10:1889–1896
Matthay KK, Yanik G, Messina J, Quach A, Huberty J, Cheng SC, Veatch J, Goldsby R, Brophy P, Kersun LS, Hawkins RA, Maris JM (2007) Phase II study on the effect of disease sites, age, and prior therapy on response to iodine-131-metaiodobenzylguanidine therapy in refractory neuroblastoma. J Clin Oncol 25:1054–1060
Conflict of interest
The authors D. Volterrani, F. Orsini, S. Chiacchio, L. Bodei declare that they have no conflict of interest.
Human and animal studies
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Color figures online at http://link.springer.com/article/10.1007/s40336-013-0043-x.
Rights and permissions
About this article
Cite this article
Volterrani, D., Orsini, F., Chiacchio, S. et al. Multiagent targeting of neuroendocrine neoplasms. Clin Transl Imaging 1, 407–421 (2013). https://doi.org/10.1007/s40336-013-0043-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-013-0043-x