Abstract
Objective
To evaluate whether pretreatment metabolic parameters obtained from positron emission tomography (PET) with [18F]fluorodeoxyglucose (FDG) can improve risk prediction for patients with oropharyngeal squamous cell carcinoma (OPSCC) treated with definitive intensity-modulated radiation therapy (IMRT).
Methods
Between 2003 and 2009, 86 patients with OPSCC had FDG-PET/CT prior to treatment with definitive IMRT. Chemotherapy was administered to 90 % of the patients. Metabolic tumor volume (MTV), total lesion glycolysis (TLG), maximum standardized uptake value (SUVmax), mean SUV (SUVmean), and inverse coefficient of variation (1/CoV) were analyzed for the primary tumor alone and the total of the primary tumor and involved lymph nodes.
Results
Median follow-up time for surviving patients was 41 months. On univariate analysis, total MTV and total TLG were significant predictors of disease-free survival (DFS) and overall survival (OS). SUVmax, SUVmean, and 1/CoV failed to predict DFS or OS. On multivariate analysis controlling for T- and N-classification, total MTV remained a significant predictor of DFS and OS. The optimal cutpoint for total MTV was 20.5 ml. A total MTV >20.5 ml was associated with a 4.13-fold increased risk of death (95 % confidence interval [CI], 2.12–8.05; p < 0.0001). Total MTV remained a significant predictor of DFS and OS for the subgroups with p16-positive (n = 25) and p16-negative (n =18) cancer.
Conclusion
Total MTV is an independent predictor of DFS and OS for patients with OPSCC treated with definitive radiotherapy. Total MTV remained predictive of DFS and OS for both p16-positive and p16-negative cancer.
Similar content being viewed by others
Introduction
Oropharyngeal squamous cell carcinoma (OPSCC) is a biologically heterogeneous disease. Human papilloma virus (HPV) positivity or p16 expression has been shown to be a strong prognostic biomarker [1–3]. The potential for risk-adapted therapy based on HPV or p16 status is the subject of an ongoing investigation. RTOG 1016 and ECOG 1308 were designed to evaluate new treatment strategies based on HPV or p16 status. Identifying additional prognostic variables in OPSCC may allow us to better define high- and low-risk groups of patients that may benefit from risk-adapted treatment strategies.
Positron emission tomography (PET) imaging with [18F]fluorodeoxyglucose (FDG) is based on tumor glucose metabolism. Identification of prognostic FDG-PET biomarkers is an area of active research. The most investigated pretreatment metabolic parameter in head and neck cancer is the maximum standardized uptake value (SUVmax), but findings have been inconsistent [4–7]. SUVmax only provides information about a single point within the tumor, and does not measure the volume or heterogeneity of metabolically active disease.
Metabolic tumor volume (MTV) is a FDG-PET parameter which quantifies the volume of metabolically active disease. Mean SUV (SUVmean) reflects the average FDG uptake of the tumor. The product of MTV and SUVmean yields the total lesion glycolysis (TLG), which provides a measure of the total activity of the tumor [8]. The inverse coefficient of variation [1/CoV, calculated as (SUVmean/standard deviation)] is a measure of tumor metabolic heterogeneity [9]. The purpose of this study was to analyze the relative prognostic value of FDG-PET parameters including MTV, SUVmax, SUVmean, TLG and 1/CoV in a cohort of patients with OPSCC treated with definitive intensity-modulated radiation therapy (IMRT). We evaluated whether the metabolic parameters of the primary tumor alone or the total of the primary tumor and all metabolically involved lymph nodes was more prognostic. Given the importance of p16 expression as a biomarker, we assessed the relationship of the most prognostic FDG-PET parameter, total MTV, to clinical outcomes in both p16-positive and p16-negative disease.
Methods
Patients
Between July 2003 and November 2009, 86 patients with newly diagnosed OPSCC were retrospectively evaluated. All patients had clinical FDG-PET/computed tomography (FDG-PET/CT) for staging prior to treatment with definitive IMRT. Clinical staging was performed according to the criteria of the American Joint Committee on Cancer (AJCC, 6th edition). Patients were excluded if they had non-carcinoma histology, evidence of distant metastatic disease at diagnosis, or had previous definitive surgery. The Washington University School of Medicine Human Research Protection Office approved this study.
FDG-PET/CT imaging
PET/CT was performed on a Siemens Biograph Duo scanner or a Siemens Biograph 40 scanner PET/CT tomography (Siemens, Knoxville, TN). These two scanners had similar performance. Patients fasted for at least 4 h, and their blood glucose levels were less than 200 mg/dl. Patients were injected intravenously with 10–15 mCi of FDG. Approximately 60 min postinjection, a spiral CT scan was obtained from the vertex to the proximal thigh. The CT consisted of a 10- to 20-s topogram acquired at 30 mAs and 110 kVp (Biograph Duo) or 35 mAs and 120 kVp (Biograph 40). Based on the topogram, the time for the CT scan was adjusted so that the effective mAs were between 95 and 111. The tube voltage remained constant at130 kVp (Biograph Duo) or 120 kVp (Biograph 40). Noncontrast CT images were obtained for attenuation correction and for fusion with PET images for lesion localization. Immediately after the CT scan, emission images were obtained, which ranged from 2 to 5 min for each of the 6–8 bed positions.
FDG-PET imaging assessment
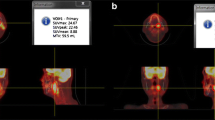
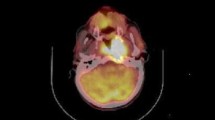
The metabolic volumes of interest were retrospectively indentified on all FDG-PET/CT scans. The MTV was defined as the tumor volume with ≥50 % of the local maximum SUV (SUVmax). The 50 % threshold used in this study has been shown to be a reliable correlate to CT tumor volume in phantom and clinical studies [10, 11]. Areas of normal physiologic FDG uptake were excluded from the threshold volumes. The MTV was defined as the volume of interest (VOI), and mean SUV (SUVmean) and standard deviation were measured for this volume. TLG was calculated as the product of SUVmean and MTV. The inverse coefficient of variation (1/CoV) was calculated as (SUVmean/SD). The MTV, SUVmax, SUVmean, TLG, and 1/CoV were determined and analyzed for the primary tumor alone and the total of the primary tumor and all metabolically involved lymph nodes. FDG-PET/CT analysis was conducted using MIMvista software (version 5.2; MIMvista Corporation, Cleveland, OH).
p16 testing
Immunohistochemistry was performed for p16 on 4-μm sections cut from formalin-fixed, paraffin-embedded tissue blocks using a monoclonal antibody to p16 (MTM Laboratories, Westborough, MA; 1:1 dilution) on a Ventana Benchmark automated immunostainer (Ventana Medical Systems, Inc., Tucson, AZ) according to standard protocols, with appropriate positive and negative controls. p16 immunostaining was considered positive if both nuclear and cytoplasmic expression was present. Staining was graded in a quartile manner as follows: 0 = negative; 1+ = 1–25 % of cells positive; 2+ = 26–50 %; 3+ = 51–75 %; 4+ = 76–100 %. For analysis, cases were divided into p16-positive (4+) or p16-negative (0 to 3+).
Clinical endpoints
Data were collected from the medical records to determine locoregional failure, distant failure, death, and time of last follow-up. Diagnosis of locoregional or distant failure required either pathologic confirmation or unequivocal clinical or radiographic evidence of failure. Disease-free survival (DFS) included any locoregional or distant failure, or death from any cause. Overall survival (OS) included death from any cause. All time intervals were calculated from the date of diagnosis.
Statistical analysis
Survival was estimated using the Kaplan–Meier method. Univariate and stepwise multivariate Cox proportional hazards models were used to evaluate the association between FDG-PET variables and DFS and OS. Logistic regression was used to evaluate the association of MTV and T- and N-classifications. Graphical diagnostic plots, the minimum p-value approach with adjusted p-values, and receiver operator characteristic (ROC) analysis were utilized to identify the optimal total MTV cutpoint with regard to OS [12]. Student’s t-test was used to analyze the effect of p16 status on the FDG-PET variables. A p value of <0.05 was considered statistically significant. All tests were two-sided. Statistical analyses were performed using StatView (version 5.0.1; SAS Institute Inc., Cary, NC) and SAS (version 9.2; SAS Institute Inc.).
Results
Patient, cancer, and treatment-related characteristics are summarized in Table 1. The median age at diagnosis was 58. Median follow-up time for surviving patients was 41 months (range 3–84 months). The majority of patients (95 %) presented with locally advanced disease (stage III–IV). The median radiation therapy dose was 70 Gy (range 66–75 Gy). The majority of patients (90 %) received chemotherapy, consisting of platinum-based chemotherapy or cetuximab. The estimated (Kaplan–Meier) 2-year and 5-year OS were 58.2 % and 35.5 %, respectively, and the 2-year and 5-year DFS were 47.6 % and 37.7 %, respectively. The estimated (Kaplan–Meier) median OS for the cohort was 39 months.
Pretreatment FDG-PET characteristics are listed in Table 2. Representative images with delineation of primary tumor MTV are shown in Fig. 1. Univariate Cox proportional hazards regression was performed to determine whether there was an association between FDG-PET variables and DFS and OS (Table 3). Primary tumor MTV was a significant predictor of DFS and OS, and primary tumor TLG was a significant predictor of OS. Total MTV and total TLG were significant predictors of DFS and OS. SUVmax, SUVmean, and 1/CoV were not significant predictors of DFS or OS.
On multivariate analysis controlling for T- and N-classifications, total MTV remained a significant predictor of DFS (hazard ratio [HR] = 1.04; 95 % confidence interval [CI], 1.02–1.06; p < 0.0001) and OS (HR = 1.03; 95 % CI, 1.01–1.05; p = 0.0005). To make this data more clinically meaningful, we sought to identify high and low risk groups based on total MTV. With regard to overall survival, the optimal cutpoint for total MTV was determined to be 20.5 ml. The optimal cutpoint was determined using the minimum p-value approach with adjusted p-values and ROC analysis [12]. A total MTV greater than 20.5 ml was significantly associated with a 4.13-fold increased risk of death (95 % CI, 2.12–8.05; p < 0.0001) (Fig. 2). On multivariate analysis controlling for T- and N-classification, total MTV >20.5 ml remained a significant predictor of DFS and OS.
Prognostic value of total MTV by p16 status
p16 status was available for 43 patients (50 %). Analyzing this subset, p16-negative cancer was associated with a worse DFS (HR = 2.59; 95 % CI, 1.15–5.84; p = 0.022) and OS (HR = 2.72; 95 % CI, 1.18–6.28; p = 0.019). Pretreatment FDG-PET characteristics for the p16-positive and p16-negative subgroups are listed in Table 2. There was no significant difference in the FDG-PET parameters between the p16-positive and p16-negative subgroups. In the subgroup of patients with p16-positive cancer (n = 25), total MTV remained a significant predictor of DFS (HR = 1.05; 95 % CI, 1.01–1.10; p = 0.028) and OS (HR = 1.05; 95 % CI, 1.01–1.10, p = 0.035). Similarly, for the subgroup of patients with p16-negative cancer (n = 18), total MTV was predictive of DFS (HR = 1.05; 95 % CI, 1.01–1.09; p = 0.032) and OS (HR = 1.05; 95 % CI, 1.01–1.10; p = 0.023).
Applying the optimal cutpoint value that was determined for the entire cohort, a total MTV >20.5 ml was associated with a 13.0-fold increased risk of death (95 % CI, 1.62–100; p = 0.016) for the p16-positive subgroup, compared to a 4.27-fold increased risk of death (95 % CI, 1.28–14.3; p = 0.018) for the p16-negative subgroup (Fig. 3).
Discussion
We report the results of a study evaluating prognostic value of pretreatment FDG-PET parameters in OPSCC. To our knowledge, this is the first report analyzing FDG-PET parameters in both p16-positive and p16-negative disease. Identification of possible prognostic metabolic parameters using FDG-PET is an area of current investigation. A summary of selected series analyzing pretreatment FDG-PET characteristics in head and neck cancer is presented in Table 4. SUVmax is the most studied FDG-PET variable. In our study, SUVmax was not predicative of DFS or OS. While SUVmax has been reported to predict outcomes, many studies have failed to show a significant association [4–6, 13].
A correlation between total MTV and outcomes, including progression-free survival (PFS) and OS, has been reported by La et al. [11] and these findings were subsequently confirmed in a validation dataset reported by the same group [4]. In their studies, the MTV was defined using a 50 % threshold, and multiple head and neck disease sites were included. Our results indicate that total MTV is a significant prognostic factor for oropharyngeal cancer, even after controlling for T- and N-classification. Thus, total MTV does not simply act as a surrogate for stage, but serves as an independent prognostic factor.
In previous reports MTV has been determined using several methods, including visual interpretation, a minimum SUV threshold, a fixed percentage threshold of the local SUVmax, and adaptive threshold methods [14]. Visual interpretation of FDG-PET is subject to window-level settings and interobserver variability [15, 16]. A threshold method allows for automated segmentation, limiting interobserver variability. A 50 % threshold of maximum SUV has been shown to be a reliable correlate to CT tumor volume in phantom and clinical studies [10, 11].
SUVmean was not a significant predictor of DFS or OS in our study. Higgins et al. [13] reported that primary tumor SUVmean was associated with worse DFS. Differences in their study compared to the present study include: (1) multiple head and neck subsites were included, as opposed to oropharynx only, (2) SUVmean was determined for a tumor volume contoured on CT, as opposed to the MTV, and (3) median follow-up time was 15 months, compared to 41 months. One or more of these differences may explain why SUVmean was not a significant predictor of outcomes in our study.
TLG measures the total activity of the tumor. Moon et al. [17] reported that primary tumor TLG was an independent predictor of overall survival, whereas primary tumor MTV was not. The involved lymph nodes were not analyzed. In our study, we analyzed the prognostic significance of the FDG-PET characteristics for the primary tumor alone, or in combination with all of the metabolically involved lymph nodes. Based on our results, while the primary tumor TLG was a significant predictor of survival on univariate analysis, the total MTV of the primary tumor and all metabolically involved lymph nodes was the most robust predictor of outcomes.
Intratumoral heterogeneity in FDG uptake may be due to necrosis, hypoxia, cellular proliferation or blood flow [9]. Intratumoral FDG metabolic heterogeneity is a poor prognostic factor for cervical cancer [18] and sarcoma [19]. In the present study, intratumoral heterogeneity, as measured by 1/CoV, was not a significant predictor of DFS or OS.
Given the significant difference in prognosis between p16-positive and p16-negative oropharyngeal cancer, does total MTV serves as a potential biomarker for both of these groups? Tang et al. [4] found that total MTV predicted PFS and OS for p16-positive oropharyngeal cancer, but the limited number of patients with p16-negative cancer in their study prevented an adequately powered analysis for this subgroup. In the present study, total MTV remained a significant predictor for DFS and OS for both the p16-positive and p16-negative subgroups. There was no significant difference in mean MTV between the p16-positive and p16-negative subgroups. Using the optimal cutpoint value that was determined for the entire cohort, we found that a total MTV >20.5 ml was significantly associated with a 13.0-fold increased risk of death in p16-positive patients and 4.27-fold increased risk of death in p16-negative patients. Given the limited number of patients with p16 status available, further investigation to define the relative prognostic value of total MTV for p16-positive and p16-negative disease is recommended.
In the present study, we limited our analysis to patients with oropharyngeal cancer. Most of the studies listed in Table 4 included multiple head and neck cancer subsites. The pathophysiology, clinical behavior, and prognosis of malignancies in these subsites can differ significantly. By limiting our analysis to OPSCC, we eliminated this potentially confounding effect.
Potential limitations of this study include its single-institution retrospective design, non-uniform treatment, and the limited number of patients with p16 status available. Despite these limitations, total MTV was highly predictive of recurrence and survival for the entire cohort and both the p16-positive and p16-negative subgroups. Ideally, the prognostic value of total MTV and other FDG-PET parameters should be validated in a prospective study.
Conclusion
Total MTV is an independent predictor of DFS and OS for patients with OPSCC treated with definitive radiotherapy. Total MTV >20.5 ml was associated with a 4.13-fold increased risk of death. Total MTV remained predictive of DFS and OS for both p16-positive and p16-negative cancer.
References
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363(1):24–35. doi:10.1056/NEJMoa0912217
Lassen P, Eriksen JG, Krogdahl A, Therkildsen MH, Ulhoi BP, Overgaard M, Specht L, Andersen E, Johansen J, Andersen LJ, Grau C, Overgaard J (2011) The influence of HPV-associated p16-expression on accelerated fractionated radiotherapy in head and neck cancer: evaluation of the randomised DAHANCA 6&7 trial. Radiother Oncol 100(1):49–55. doi:10.1016/j.radonc.2011.02.010
Posner MR, Lorch JH, Goloubeva O, Tan M, Schumaker LM, Sarlis NJ, Haddad RI, Cullen KJ (2011) Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol 22(5):1071–1077. doi:10.1093/annonc/mdr006
Tang C, Murphy JD, Khong B, La TH, Kong C, Fischbein NJ, Colevas AD, Iagaru AH, Graves EE, Loo BW Jr, Le QT (2012) Validation that metabolic tumor volume predicts outcome in head-and-neck cancer. Int J Radiat Oncol Biol Phys 83(5):1514–1520. doi:10.1016/j.ijrobp. 2011.10.023
Schwartz DL, Rajendran J, Yueh B, Coltrera MD, Leblanc M, Eary J, Krohn K (2004) FDG-PET prediction of head and neck squamous cell cancer outcomes. Arch Otolaryngol Head Neck Surg 130(12):1361–1367. doi:10.1001/archotol.130.12.1361
Seol YM, Kwon BR, Song MK, Choi YJ, Shin HJ, Chung JS, Cho GJ, Lee JC, Lee BJ, Wang SG, Kim HJ, Kim WT, Kim SJ, Yun EY (2010) Measurement of tumor volume by PET to evaluate prognosis in patients with head and neck cancer treated by chemo-radiation therapy. Acta Oncol 49(2):201–208. doi:10.3109/02841860903440270
Kubicek GJ, Champ C, Fogh S, Wang F, Reddy E, Intenzo C, Dusing RW, Machtay M (2010) FDG-PET staging and importance of lymph node SUV in head and neck cancer. Head Neck Oncol 2:19. doi:10.1186/1758-3284-2-19
Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, Casilla C, Fazzari M, Srivastava N, Yeung HW, Humm JL, Guillem J, Downey R, Karpeh M, Cohen AE, Ginsberg R (1999) Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging 2(3):159–171
van Velden FH, Cheebsumon P, Yaqub M, Smit EF, Hoekstra OS, Lammertsma AA, Boellaard R (2011) Evaluation of a cumulative SUV-volume histogram method for parameterizing heterogeneous intratumoural FDG uptake in non-small cell lung cancer PET studies. Eur J Nucl Med Mol Imaging 38(9):1636–1647. doi:10.1007/s00259-011-1845-6
Ciernik IF, Dizendorf E, Baumert BG, Reiner B, Burger C, Davis JB, Lutolf UM, Steinert HC, Von Schulthess GK (2003) Radiation treatment planning with an integrated positron emission and computer tomography (PET/CT): a feasibility study. Int J Radiat Oncol Biol Phys 57(3):853–863
La TH, Filion EJ, Turnbull BB, Chu JN, Lee P, Nguyen K, Maxim P, Quon A, Graves EE, Loo BW Jr, Le QT (2009) Metabolic tumor volume predicts for recurrence and death in head-and-neck cancer. Int J Radiat Oncol Biol Phys 74(5):1335–1341. doi:10.1016/j.ijrobp. 2008.10.060
Williams BA, Mandrekar JN, Mandrekar SJ, Cha SS, Furth AF (2006) Finding optimal cutpoints for continuous covariates with binary and time-to-event outcomes. Technical Report Series No 79. Department of Health Science Research, Mayo Clinic, Rochester
Higgins KA, Hoang JK, Roach MC, Chino J, Yoo DS, Turkington TG, Brizel DM (2012) Analysis of pretreatment FDG-PET SUV parameters in head-and-neck cancer: tumor SUVmean has superior prognostic value. Int J Radiat Oncol Biol Phys 82(2):548–553. doi:10.1016/j.ijrobp. 2010.11.050
Schinagl DA, Vogel WV, Hoffmann AL, van Dalen JA, Oyen WJ, Kaanders JH (2007) Comparison of five segmentation tools for 18F-fluoro-deoxy-glucose-positron emission tomography-based target volume definition in head and neck cancer. Int J Radiat Oncol Biol Phys 69(4):1282–1289. doi:10.1016/j.ijrobp. 2007.07.2333
Riegel AC, Berson AM, Destian S, Ng T, Tena LB, Mitnick RJ, Wong PS (2006) Variability of gross tumor volume delineation in head-and-neck cancer using CT and PET/CT fusion. Int J Radiat Oncol Biol Phys 65(3):726–732. doi:10.1016/j.ijrobp. 2006.01.014
Gregoire V, Bol A, Geets X, Lee J (2006) Is PET-based treatment planning the new standard in modern radiotherapy? The head and neck paradigm. Semin Radiat Oncol 16(4):232–238. doi:10.1016/j.semradonc.2006.04.006
Moon SH, Choi JY, Lee HJ, Son YI, Baek CH, Ahn YC, Park K, Lee KH, Kim BT (2012) Prognostic value of (18) F-FDG PET/CT in patients with squamous cell carcinoma of the tonsil: Comparisons of volume-based metabolic parameters. Head Neck. doi:10.1002/hed.22904
Kidd EA, Grigsby PW (2008) Intratumoral metabolic heterogeneity of cervical cancer. Clin Cancer Res 14(16):5236–5241. doi:10.1158/1078-0432.CCR-07-5252
Eary JF, O'Sullivan F, O'Sullivan J, Conrad EU (2008) Spatial heterogeneity in sarcoma 18F-FDG uptake as a predictor of patient outcome. J Nucl Med 49(12):1973–1979. doi:10.2967/jnumed.108.053397
Chung MK, Jeong HS, Park SG, Jang JY, Choi JY, Hyun SH, Park K, Ahn MJ, Ahn YC, Kim HJ, Ko YH, Baek CH (2009) Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res 15(18):5861–5868. doi:10.1158/1078-0432.CCR-08-3290
Conflict of interest
The authors declare that no actual or potential conflicts of interest exist.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garsa, A.A., Chang, A.J., DeWees, T. et al. Prognostic value of 18F-FDG PET metabolic parameters in oropharyngeal squamous cell carcinoma. J Radiat Oncol 2, 27–34 (2013). https://doi.org/10.1007/s13566-012-0065-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13566-012-0065-4